Scheme 1.
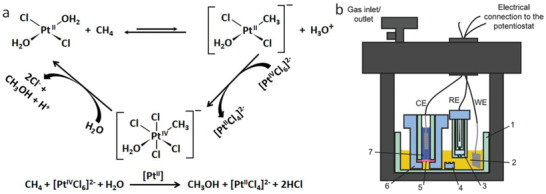
a) Shilov cycle and overall chemical reaction for electrochemical selective oxidation of methane to methanol. b) The diagram of the electrochemical cell used for methane oxidation by Surendranath et al. WE: working electrode, Pt foil; RE: reference electrode, Ag/AgCl; CE: counter electrode, Pt mesh. 1: glass cell; 2: reaction solution containing Pt salt; 3: fritted tube for making room of RE; 4: PTFE stir bar; 5: H+ ion conducting membrane; 6: PTFE body holding the membrane stack and 7: counter electrode compartment containing sacrificial electron acceptor VOSO4 (3 m). The WE compartment contained 3 × 10−3 m K2PtCl4, 7 × 10−3 m Na2PtCl6⋅6H2O, 10 × 10−3 m NaCl, and 0.5 m H2SO4. The cell and solution were O2‐free and pressurized with CH4 at 500 psi and the temperature was 130 °C. Reproduced with permission.[ 130 ] Copyright 2019, American Chemical Society (https://pubs.acs.org/doi/10.1021/acscentsci.9b00273, further permission related to the material excerpted should be directed to the ACS).
