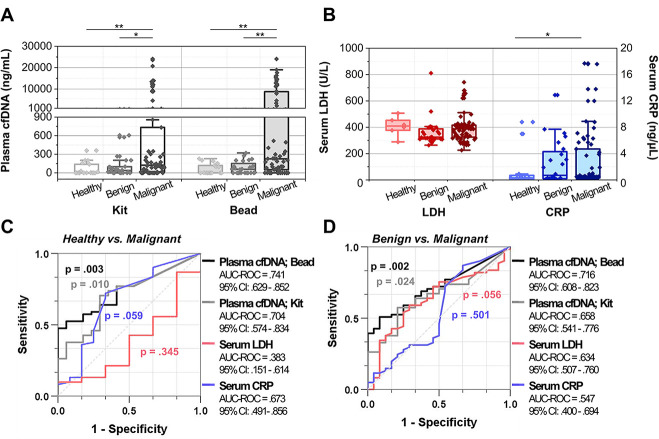
Fig 2. Plasma cfDNA and serum antigen levels quantified from a total of 85 patients with gastric tumor, 61 with malignant tumor and 24 with benign tumor, and 17 healthy individuals.
(a) The amount of plasma cfDNA levels measured from each cohort using the new bead-based cfDNA separation system and commercially available QIAamp DNA mini kit. (b) The amount of serum LDH and CRP levels measured from each cohort. (c, d) The diagnostic capability of the new cfDNA separation platform compared with commercially available QIAamp DNA mini kit and other serum antigen blood tests. The new bead-based system demonstrated the highest performance in distinguishing the patients with malignant tumor from both (c) healthy donors and (d) patients with benign tumor.