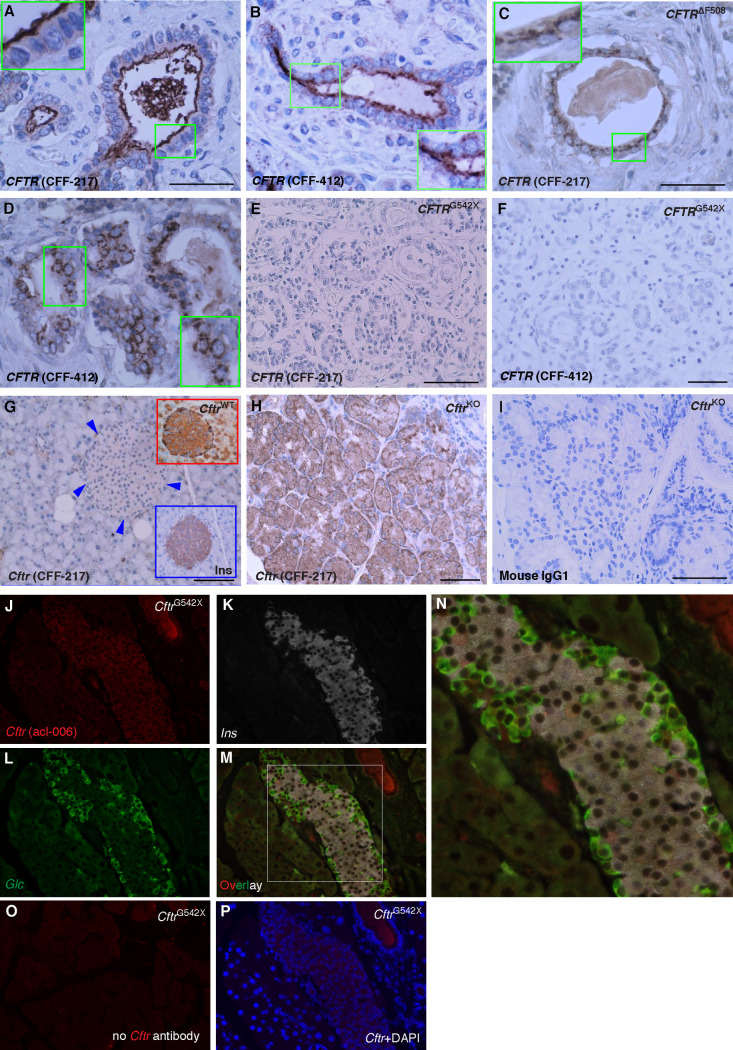
Fig 2. CFTR antibody validation.
A-D. Shown are microscopy images of human pancreas from a normal donor (A, B, 11-month-old control) or homozygous for the CFTR mutation F508del (C, D, 6-month-old CF patient) immunostained using CFF-217 (A, C) or CFF 412 (B, D). Note the expected apical and intracellular immunolabeling of CFTR in normal and mutant pancreas, respectively. The insets indicate higher magnification. E, F. Microscopy images of human pancreas from a 1 yr. old CF patient homozygous for CFTRG542X using the antibodies CFF-217 (E) and CFF-412 (F). Shown is absence of immunostaining with both antibodies. G-I. Microscopy images of pancreas (G) and intestine (H) of CftrKO mice immunolabeled using CFF-217. Shown are the expected patterns of immunostaining for specific antibodies. For comparison, shown are magnified images of CftrWT pancreas islets using CFTR and insulin antibodies (top and bottom right corners in G, respectively). I. Omission of the CFTR antibody did not generate immunostaining in the intestine. Bars in A-I represents 50μm. J-L. Pancreatic tissue from CftrG542X mice co-immunolabeled by using the indicated mouse CFTR-specific antibody acl-006 (J), insulin (K, Ins) and glucagon (L, Glc). M-N. Overlay image of J-L and magnified squared area shown in N. O-P. Control images obtained from CftrG542X mice in the absence of primary (O) or secondary antibodies (P). DAPI was used to counterstain nuclei in P.