Abstract
Quality of life (QoL) is a matter of concern in both healthy and diseased individuals. Lifestyle factors such as physical activity and sleep have a direct impact on QoL. In this context, interactions between activity time expenditure and QoL might be different in comorbid and non comorbid patients. Besides, the quantification and evaluation of time expenditure is ordinarily measured as the absolute time devoted to each activity. The objective of this study is the evaluation of the influence and interactions of activity-relative time expenditure and co-morbidity in Physical QoL.
The study involved 302 consecutive patients, from an Internal Medicine ambulatory evaluation. Validated questionnaires were used to collect demographic variables and time expenditure variables. QoL was gathered with de survey short form-36questionnaire. Comorbidity was compiled with de Charlson Comorbidity Index. SPSS v20.0 was used for statistical analysis.
As hypothesized, healthy subjects had higher Physical QoL score than comorbid subjects (P < .05). Physical activity and sleep relative time expenditure were statistically significant and associated to a better QoL in comorbid patients (P < .05). Interestingly, sleep was found to have statistically significant interaction with a score of ≥2 in the Charlson Comorbidity Index. Age, gender, comorbidity, physical activity relative time expenditure, and the interaction between relative time dedicated to sleep and comorbidity were found statistically significant in a multivariate model on Physical QoL prediction.
Activity-relative time expenditure could be an adequate measure of daily activity pattern in the evaluation of QoL. Relative time spent in physical activity and sleep might be positively associated to Physical QoL. Sleep and comorbidity could have a statistically significant interaction in the prediction of Physical QoL.
Keywords: lifestyle, morbidity, physical activity, quality of life
1. Introduction
Quality of life (QoL) has been defined by the World Health Organization as “the perception of the position in life of individuals in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards, and concerns’.”[1] This concept of QoL is a concise resume of multiple interactions, since different studies evidenced the importance of quantifying health issues such as disease, lifestyle, sleep, nutrition, and genetics in wellbeing.[2–4]
The assessment of QoL commonly relies on different questionnaires and surveys, which have been applied to healthy and diseased populations. Most of them, such as survey short form-36 (SF-36), European quality of life scale, and World Health Organization quality of life assessment, provide a standardized measure from personal information and subjective queries about life quality through validated questionnaires.[1,5,6] In this context, SF-36 is considered a valid and reliable test in both investigation and clinical practice to measure physical and mental QoL. This kind of survey has been used in research of multiple pathological conditions such as diabetes, heart disease, cancer, asthma, renal disease, and multi-morbidity.[7–12]
Indeed, morbidities have an influence in QoL in different degrees, depending on illness severity, chronicity, and symptoms clustering.[12–14] However, the assessment of co-morbidity could be a difficult task because of the heterogeneity of illnesses. In this context, different scales have been developed in order to predict survival depending on accumulation of chronic disease. Among these, the Charlson comorbidity index (CCI) appears to be an outstanding tool to evaluate disease burden, with a worldwide validation and practical application.[15]
Lifestyle is another factor influencing QoL. Since, nutritional status and dietary intake as well as sleep and physical activity patterns have shown an impact over mental and physical health issues involving overall wellbeing status.[16–18] Besides, the relationship between co-morbidity and lifestyle has been widely described.[19] Thus, multiple interactions have been proven between disease and nutrition, sleep or physical activity and usually tend to be evaluated as single data.[20–22]
This approach may lose the holistic evaluation of activity time expenditure. As time is limited, different activities interact with each other. In this context, relative evaluation of time expenditure, defined as the percentage of time devoted to each activity divided by the entire declared time, could also be a precise method of evaluating the influence of different activities on well-being. Additionally, this strategy could provide a real-life based medical advice as well as an additional adjustment in terms of epidemiological analysis.
Accordingly, the objective of our study is to evaluate the interaction between the weekly activity pattern measured in relative time devotion to 5 main activities (Working time, active leisure time, physical activity, sleep and time of sedentarism), and co-morbidity in the prediction of Physical QoL according to SF-36.
2. Patients and methods
The study involved 302 consecutive in coming patients, who attended to an Internal Medicine ambulatory evaluation in a Spanish tertiary hospital between October 2018 and March 2019 and filled a validated questionnaire including lifestyle variables[23] and SF-36 v2 form.[6] This survey recorded demographic data and time devotion to work, active leisure, physical activity, sleep, and sedentarism as well as diverse clinical information. The attending physician used the CCI to collect morbidities.[15] The study was approved by the center bioethics committee (ESCAVIDA/04). Of this cohort, 262 individuals (86.72%) fulfilled the complete questionnaire and their data were analyzed in the study.
Standardized coefficients were used to turn the 8 SF-36 categories into the Physical and the Mental SF-36 summaries.[24] Total declared activity time was calculated for each patient as the sum of the time devoted to work, leisure, physical activity, sleep, and sedentary activities. Subgroups of activity related time expenditure (ArTE) were obtained dividing time expenditure in each activity and total declared activity time. Patients were considered co-morbid when they scored ≥2 in CCI.
Conventional statistical tests, including Chi-square and T-Student were applied as appropriate. Factorial 2x2 ANOVA analysis were performed to evaluate interactions between comorbidity and ArTE. Multivariate regression models were ran considering Physical QoL as a dependent variable, while confounding and adjusting variables, such as age or sex, were also fitted in the model. To avoid co-linearity, age was included separately in the multivariate models and was not considered as part of CCI. Results were considered statistically significant with a P-value < .05. The IBM SPSS statistical package v20.0 (Chicago, 2011) was used to perform the analysis.
3. Results
The study population presented a mean age of 57 ± 17 years. Female participants accounted the 52% of the sample. Different diseases were found in 89 patients, where, according to the CCI, were distributed as follows: Acute myocardial infarction (7.28%), heart failure (3.31%), peripheral artery disease (7.61%), cerebrovascular disease (7.94%), low grade dementia (2.58%), chronic obstructive pulmonary disease (8.94%), connective tissue disease (9.93%), mild liver disease (5.63%), diabetes mellitus (9.93%), renal failure (3.97%), localized solid organ tumors (8.28%), leukemia (0.33%), liver cirrhosis (0.99%), and metastatic cancer disease (1.32%). CCI mean was 1.04 ± 1.5 comorbidities. Besides, 76 patients were found to have a CCI ≥2 (25.16%). As expected, after stratifying the sample in 2 groups depending on age (Cutoff 65 years), statistically significant differences were found between patients in all co-morbidities with the exception of liver disease and cancer, in the total comorbidities and in the Charlson comorbidity index (Table 1).
Table 1.
Population characteristics at entry concerning demographic variables and co-morbidity categorized by age.
| Variable (n = 302) | Mean (SD) n (%) | Age <65 n = 184 | Age >65 n = 118 | P |
| Age, years | 57.66 (17.87) | 46.19 (12.42) | 75.53 (7.15) | .01 |
| Gender, female | 161 (51.93%) | 103 (55.98%) | 54 (45.76%) | .08 |
| Co-morbidities | ||||
| Acute myocardial infarction | 22 (7.28%) | 1 (0.54%) | 21 (17.79%) | .01 |
| Heart failure | 10 (3.31%) | 0 (0%) | 10 (8.47%) | .01 |
| Peripheral artery disease | 23 (7.61%) | 9 (4.89%) | 14 (11.86%) | .03 |
| Cerebrovascular disease | 24 (7.94%) | 9 (4.89%) | 15 (12.71%) | .01 |
| Dementia | 8 (2.58%) | 1 (0.54%) | 7 (5.93%) | .01 |
| COPD | 27 (8.94%) | 4 (2.17%) | 23 (19.49%) | .01 |
| Connective tissue disease | 30 (9.93%) | 13 (7.06%) | 17 (14.41%) | .04 |
| Mild liver disease | 17 (5.63%) | 11 (5.98%) | 6 (5,.08%) | .74 |
| Diabetes mellitus (no organ damage) | 26 (8.61%) | 7 (3.80%) | 19 (16.10%) | .01 |
| Diabetes mellitus (organ failure) | 4 (1.32%) | 0 (0%) | 4 (3.39%) | .01 |
| Renal failure | 12 (3.97%) | 0 (0%) | 12 (10.17%) | .01 |
| Localized solid organ tumor | 25 (8.28%) | 11 (5.98%) | 14 (11.86%) | .07 |
| Leukemia | 1 (0.33%) | 0 (0%) | 1 (0.84%) | .21 |
| Liver cirrhosis | 3 (0.99%) | 1 (0.54%) | 2 (1.69%) | .32 |
| Metastatic cancer | 4 (1.32%) | 3 (1.63%) | 1 (0.84%) | .56 |
| Comorbidity groups | ||||
| No comorbidities | 152 (50.32%) | 124 (67.39%) | 28 (23.7%) | .01 |
| One comorbidity | 89 (29.47%) | 47 (25.54%) | 42 (35.6%) | |
| Comorbid patients | 61 (20.21%) | 13 (7.07%) | 48 (40.7%) | |
| Total co-morbidities | 0.82 (1.08) | 0.41 (0,65) | 1.46 (1.28) | .01 |
| Charlson comorbidity index | 1.04 (1.50) | 0.56 (1.15) | 1.80 (1.66) | .01 |
| Charlson comorbidity index ≥ 2 | 76 (25.16) | 21 (11.41) | 55 (46.51) | .01 |
QoL, as measured by SF-36 provides an 8-category evaluation. When classifying the sample depending on comorbidity, all categories, with the exception of mental QoL, emotional role, and corporal pain were statistically significant and lower in CCI ≥2 patients (Table 2).
Table 2.
Quality of life characteristics concerning the 8 categories of SF-36 and boty physical and mental summaries categorized by comorbidity.
| Categories | Global n = 262 | CCI <2 n = 204 | CCI ≥2 n = 58 | P |
| General (%) | 56.01 (20.04) | 59.28 (18.29) | 44,.48 (21.76) | .01 |
| Physical (%) | 79.88 (25.73) | 85.37 (19.58) | 60.60 (34.36) | .01 |
| Physical role (%) | 79.17 (26.01) | 81.95 (24.48) | 69.39 (28.98) | .01 |
| Mental (%) | 70.74 (19.71) | 71.67 (19.11) | 67.50 (21.76) | .16 |
| Emotional role (%) | 86.25 (22.17) | 86.27 (21.81) | 86.21 (23.60) | .70 |
| Corporal pain (%) | 65.88 (25.54) | 67.30 (25.18) | 60.91 (26.40) | .16 |
ArTE encompassed 5 activities: work, leisure time, physical activity, sleep, and sedentarism with a mean of 22.78 hours, 11.08 hours, 4.30 hours, 45.49 hours, and 37.88 hours per week, respectively. To provide an adjusted evaluation of time expenditure, these variables were converted to relative values by estimating the percentage of time devoted to each activity. These calculations were made by dividing the time spent in an activity by the sum of the reported time devoted to the 5 activities accounting ArTE. After comparing patients time expenditure depending on comorbidity status (CCI <2 vs CCI ≥2), healthier patients tended to spend more time at work and less time sleeping or devoted to sedentary activities (P < .05). No statistically significant differences were found in time devoted to leisure or physical activity (Table 3).
Table 3.
Time spent (hours/week and time percentage distribution of time expenditure) on different activities categorized by comorbidity status.
| Week time | % of total time | ||||
| Activity | Hours/week (SD) | % of time (SD) | CCI <2 | CCI ≥2 | P |
| Working time | 22.78 (21.68) | 16.46% (15.28%) | 19.07% (15.21%) | 7.29% (11.58%) | .01 |
| Leisure time | 11.08 (13.04) | 8.59% (9.27%) | 8.77% (9.31%) | 7.97% (9.20%) | .56 |
| Physical activity | 4.30 (6.22) | 3.75% (5.48%) | 3.70% (4.92%) | 3.92% (7.16%) | .78 |
| Sleep | 45.49 (10.25) | 41.82% (17.14%) | 40.44% (16.76%) | 46.67% (17.74%) | .01 |
| Sedentarism | 37.88 (28.16) | 29.37% (17.90%) | 28.01% (17.90%) | 34.15% (17.21%) | .02 |
Then, interactions between time expenditure subgroups and comorbidity in the prediction of Physical QoL were evaluated. To perform these analyses, activity subgroups were categorized by the median in less and more activity groups. Physical activity was found to be significantly different in both healthy (Less physical activity 63.05% ± 22.46% versus more physical activity 74.43 ± 16.19; P = .01) and diseased individuals (Less physical activity 45.08 ± 24.77 vs 56.38 ± 22.95 more physical activity; P = .01). However, no interaction was proven between CCI and physical activity (P for interaction = .99). Interestingly, relative sleep time was associated with a worse Physical QoL in healthier individuals while correlated with a better QoL in co-morbid patients (CCI <2: Less sleep 71.71 ± 19.14 vs more sleep 66.80 ± 20.53, P = .01; CCI ≥2: Less sleep 36.15 ± 17.21 vs more sleep 56.67 ± 24.81, P = .01). Indeed, a statistically significant interaction was found between comorbidity and sleep (P for interaction = .01). No other variables appeared to have statistically significant capacity of prediction of Physical QoL. Results from interactions of time expenditure subgroups are shown (Fig. 1).
Figure 1.
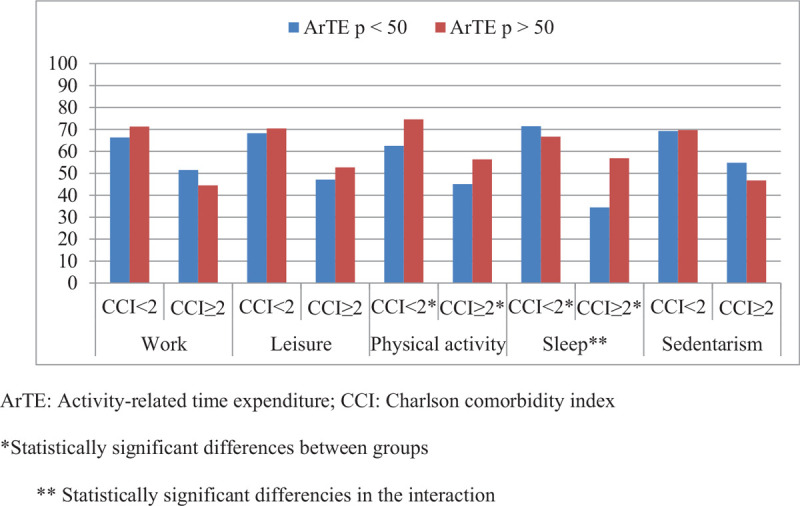
Interactions between comorbidity and time expenditure activities in physical QoL when dividing ArTE for each activity by the median. ∗Statistically significant differences between groups. ∗∗Statistically significant differences in the interaction. ArTE = activity-related time expenditure, CCI = Charlson comorbidity index.
These results allowed to fit a linear regression model of prediction of Physical QoL based on ArTE and comorbidity. In this equation, Age (B = −0.3 ± 0.1) and a Charlson comorbidity index higher than 2 (B = −30.8 ± 7.7) had a negative influence on Physical QoL. Besides, female sex, physical activity, and interaction between CCI and sleep time, were positively related to Physical QoL (B = 4.9 ± 2.5, B = 40.5 ± 15 and B = 67.7 ± 28.7, respectively). All factors were found to be statistically significant. R value for the regression was 0.47 while R2 was 0.22 (Table 4).
Table 4.
Multivariate regression model for physical QoL based on comorbidity, time expenditure, and interactions.
| QoL-physical | ||
| Variable | B (SE) | P |
| Age (yr) | −0.32 (0.08) | .01 |
| Gender (female) | 5.91 (2.50) | .02 |
| CCI ≥2 | −20.19 (4.24) | .01 |
| % Physical activity | 52.97 (22.70) | .02 |
| Interaction % sleep and CCI | 11.30 (5.80) | .05 |
4. Discussion
Achieving a better QoL in healthy subjects and patients should be a must in modern medicine, but investigations in this subject needs to be adapted to the heterogeneity of the population, with comorbidity as a cornerstone in the medical scenario.[25] The results from the present study reflect that the impact of time expenditure on QoL is different depending on the morbidity status. This statement could influence the implementation of healthy lifestyle patterns bearing in mind the relative activity expenditure approach. Besides, this current study may reinforce the value and importance of lifestyle patterns in personalized health assessment and could, if prospectively confirmed, determine medical advice in precision medicine.
The recruitment of the population of the study from a medical visit is another interesting point of our research. Lifestyle recommendations tend to be based on the analysis of healthy individuals and released through Public Health channels.[26] However, the development of QoL assessment in clinical practice needs to be supported by real life data.[27,28] Besides, general health questionnaires tend to be burdensome and difficult to interpret in the medical setting. The high adherence to the questionnaire (>85%), the plausibility of our results, and the conclusions obtained in our population could increase the interest of our research and launch the progress on individualized counseling on lifestyle modifications in the outpatient medical scenario.
The questionnaires applied for QoL and co-morbidities have both been validated to be used in the Spanish population and well recognized to be implemented in different diseases.[29,30] The lifestyle and well being questionnaire was specifically designed to analyze the impact of nutrition, physical activity, and disease on global health.[23]
A peer review of the general characteristics of the population, reveals that the prevalence of disease followed the expected trends, although in some cases, the results did not achieve statistical differences which could be attributed to the size of the sample. Furthermore, the CCI ≥2 as a cut off to define co-morbid status has previously been used in the scientific literature.[31–33] Besides, the outcomes concerning the analysis of the SF-36 domains confirmed that a higher co-morbidity was associated to poorer Physical QoL values, while the impact on Mental QoL was less evident.
A new perspective included in this study was to reflect the time devoted to different activities as part of an all activity related time expenditure value. This method (abbreviated as ArTE), consists in using the percentage of week time devoted to one of the following activities: Work, leisure, physical activity, sleep, and sedentarism as predictive variables of Physical QoL. This relative approach has been widely applied to analyze the different kilocalorie percentage of the energy contributed by each macronutrient instead of the absolute grams.[34] The evaluation of activity patterns instead of time expenditure forms alone could provide a comprehensive view of individuals time expenditure and the interrelations between different activities. Furthermore, this method could reduce confusion factors by including all activities in the evaluation and may allow a broader medical counseling in time devotion in order to achieve a better Physical QoL.
The evaluation of the different activities was interpreted as follows. On the one hand, plausible results were found when evaluating physical activity. In fact, the positive association between physical activity and Physical QoL are in accordance to current literature and contribute to the consistency of our investigation.[35,36] On the other hand, interesting results were found when evaluating relative sleep time in both healthy and comorbid individuals. Sleep time is usually related to a better QoL.[37] In this context, the results of our study could lead to the wrong conclusion of considering that sleep worsens the Physical QoL in healthy individuals. The adequate interpretation of our data would be that healthy individuals might benefit of the expenditure of time in other neutral or positive activities in terms of Physical QoL to improve their wellbeing, while diseased individuals would take advantage of reducing other activities in order to gain sleep time. The interaction between comorbidity and relative sleep time could be the cornerstone of a new gain in precise medical advice. This study could help to define the pivotal role of sleep depending on comorbidity and adjusted by age, sex, CCI, and physical activity in the prediction of Physical QoL, although these results should be confirmed in prospective studies.
The present study has some limitations. Although the sample size is enough to detect important differences of Physical QoL as demonstrated by retrospective calculation of sample size, our cohort is small in comparison to epidemiological cohorts. The cross-sectional design of this investigation does not permit the assessment of causality. Nevertheless, the broad validation of the used scales, together with the plausibility and consistency of the results might reinforce the value to understand the association between sleep, Physical QoL, and comorbidity.
In synthesis, the ArTE approach to time expenditure could simplify the evaluation of daily activity patterns. Beyond, this method could provide a more accurate intervention on daily activities of patients by reducing time devotion to negative or neutral activities in terms of Physical QoL by positive activities in this context. Besides, the interaction between comorbidity and sleep may permit to individualize medical counseling according to disease burden.
Author contributions
Conceptualization: Rafael Suarez-Villar, Diego Martinez-Urbistondo, Maria Lopez-Cano, Eva Fernandez, Laura Prosper, Paula Villares, Jose Alfredo Martinez.
Data curation: Rafael Suarez-Villar, Diego Martinez-Urbistondo, Maria Lopez-Cano, Eva Fernandez, Andrea Dominguez, Laura Prosper, Ana Rodriguez-Cobo, Maria Elena Caro Tinoco, Paula Nadal, Carlos Risco Risco, Paula Villares, Jose Alfredo Martinez.
Formal analysis: Diego Martinez-Urbistondo, Jose Alfredo Martinez.
Funding acquisition: Jose Alfredo Martinez.
Investigation: Rafael Suarez-Villar, Diego Martinez-Urbistondo, Jose Alfredo Martinez.
Methodology: Rafael Suarez-Villar, Diego Martinez-Urbistondo, Maria Lopez-Cano, Eva Fernandez, Laura Prosper, Paula Villares, Jose Alfredo Martinez.
Project administration: Rafael Suarez-Villar, Diego Martinez-Urbistondo, Paula Villares, Jose Alfredo Martinez.
Resources: Diego Martinez-Urbistondo, Paula Villares, Jose Alfredo Martinez.
Software: Rafael Suarez-Villar, Diego Martinez-Urbistondo, Jose Alfredo Martinez.
Supervision: Rafael Suarez-Villar, Diego Martinez-Urbistondo, Paula Villares, Jose Alfredo Martinez.
Validation: Rafael Suarez-Villar, Diego Martinez-Urbistondo, Jose Alfredo Martinez.
Visualization: Rafael Suarez-Villar, Diego Martinez-Urbistondo, Jose Alfredo Martinez.
Writing – original draft: Rafael Suarez-Villar, Diego Martinez-Urbistondo, Jose Alfredo Martinez.
Writing – review & editing: Diego Martinez-Urbistondo, Jose Alfredo Martinez.
Footnotes
Abbreviations: ArTE = activity-relative time expenditure, CCI = Charlson comorbidity index, QoL = quality of life, SF-36 = medical outcomes survey short form-36.
How to cite this article: Suarez-Villar R, Martinez-Urbistondo D, Fernandez MA, Lopez-Cano M, Fernandez E, Dominguez A, Prosper L, Rodriguez-Cobo A, Tinoco ME, Nadal P, Risco CR, Fernández PV, Martínez JA. Cross-sectional evaluation of the interaction between activity relative-time expenditure and comorbidity concerning physical quality of life. Medicine. 2020;99:48(e22552).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
CCI = Charlson comorbidity index, COPD = chronic obstructive pulmonary disease, SD = standard deviation.
CCI = Charlson comorbidity index.
CCI = Charlson comorbidity index, SD = standard deviation.
CCI = Charlson comorbidity index, QoL = quality of life.
References
- [1].The World Health Organization Quality of Life assessment (WHOQOL): position paper from the World Health Organization. Soc Sci Med 1995;41:1403–9. [DOI] [PubMed] [Google Scholar]
- [2].Mujica-Mota RE, Roberts M, Abel G, et al. Common patterns of morbidity and multi-morbidity and their impact on health-related quality of life: evidence from a national survey. Qual Life Res 2015;24:909–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bolge SC, Doan JF, Kannan H, et al. Association of insomnia with quality of life, work productivity, and activity impairment. Qual Life Res 2009;18:415–22. [DOI] [PubMed] [Google Scholar]
- [4].Galilea-Zabalza I, Buil-Cosiales P, Salas-Salvadó J, et al. Mediterranean diet and quality of life: Baseline cross-sectional analysis of the PREDIMED-PLUS trial. PLoS One 2018;13:e0198974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].EuroQol Group EuroQol--a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- [6].Brazier JE, Harper R, Jones NM, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ 1992;305:160–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bjorner JB, Lyng Wolden M, Gundgaard J, et al. Benchmarks for interpretation of score differences on the SF-36 health survey for patients with diabetes. Value Health 2013;16:993–1000. [DOI] [PubMed] [Google Scholar]
- [8].Moradi M, Daneshi F, Behzadmehr R, et al. Quality of life of chronic heart failure patients: a systematic review and meta-analysis. Heart Fail Rev 2019;doi: 10.1007/s10741-019-09890-2. [DOI] [PubMed] [Google Scholar]
- [9].Bunevicius A. Reliability and validity of the SF-36 health survey questionnaire in patients with brain tumors: a cross-sectional study. Health Qual Life Outcomes 2017;15:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Boussoffara L, Keskes Boudawara N, Loukil M, et al. Asthma control and quality of life. Rev Pneumol Clin 2017;73:225–30. [DOI] [PubMed] [Google Scholar]
- [11].Rogan A, McCarthy K, McGregor G, et al. Quality of life measures predict cardiovascular health and physical performance in chronic renal failure patients. PLoS One 2017;12:e0183926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Makovski TT, Schmitz S, Zeegers MP, et al. Multimorbidity and quality of life: systematic literature review and meta-analysis. Ageing Res Rev 2019;53:100903. [DOI] [PubMed] [Google Scholar]
- [13].González-Chica DA, Hill CL, Gill TK, et al. Individual diseases or clustering of health conditions? Association between multiple chronic diseases and health-related quality of life in adults. Health Qual Life Outcomes 2017;15:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Vaske I, Kenn K, Keil DC, et al. Illness perceptions and coping with disease in chronic obstructive pulmonary disease: Effects on health-related quality of life. J Health Psychol 2017;22:1570–81. [DOI] [PubMed] [Google Scholar]
- [15].Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- [16].Khalesi S, Irwin C, Sun J. Dietary patterns, nutrition knowledge, lifestyle, and health-related quality of life: associations with anti-hypertension medication adherence in a sample of Australian adults. High Blood Press Cardiovasc Prev 2017;24:453–62. [DOI] [PubMed] [Google Scholar]
- [17].St-Onge MP, Grandner MA, Brown D, et al. Sleep duration and quality: impact on lifestyle behaviors and cardiometabolic health: a scientific statement from the American Heart Association. Circulation 2016;134:e367–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Stoutenberg M, Falcon A, Arheart K, et al. Implementation of lifestyle modification program focusing on physical activity and dietary habits in a large group, community-based setting. Health Educ Behav 2017;44:421–30. [DOI] [PubMed] [Google Scholar]
- [19].Wikström K, Lindström J, Harald K, et al. Clinical and lifestyle-related risk factors for incident multimorbidity: 10-year follow-up of Finnish population-based cohorts 1982–2012. Eur J Intern Med 2015;26:211–6. [DOI] [PubMed] [Google Scholar]
- [20].Buchman AL. Nutrition and disease. Gastroenterol Clin North Am 2018;47:xiii–v. [DOI] [PubMed] [Google Scholar]
- [21].Covassin N, Singh P. Sleep duration and cardiovascular disease risk: epidemiologic and experimental evidence. Sleep Med Clin 2016;11:81–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kumar B, Robinson R, Till S. Physical activity and health in adolescence. Clin Med (Lond) 2015;15:267–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].de Cuevillas B, Álvarez Álvarez I, Cuervo M, et al. Definition of nutritionally qualitative categorizing (proto)nutritypes and a pilot quantitative nutrimeter for mirroring nutritional well-being based on a quality of life health related questionnaire. Nutr Hosp 2019;36:862–74. [DOI] [PubMed] [Google Scholar]
- [24].Bullinger M, Alonso J, Apolone G, et al. Translating health status questionnaires and evaluating their quality: the IQOLA project approach. international quality of life assessment. J Clin Epidemiol 1998;51:913–23. [DOI] [PubMed] [Google Scholar]
- [25].Fortin M, Lapointe L, Hudon C, et al. Multimorbidity and quality of life in primary care: a systematic review. Health Qual Life Outcomes 2004;2:51.doi: 10.1186/1477-7525-2-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Pérez-Martínez P, Mikhailidis DP, Athyros VG, et al. Lifestyle recommendations for the prevention and management of metabolic syndrome: an international panel recommendation. Nutr Rev 2017;75:307–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Catania G, Beccaro M, Costantini M, et al. Effectiveness of complex interventions focused on quality-of-life assessment to improve palliative care patients’ outcomes: a systematic review. Palliat Med 2015;29:5–21. [DOI] [PubMed] [Google Scholar]
- [28].Aktas A, Hullihen B, Shrotriya S, et al. Connected health: cancer symptom and quality-of-life assessment using a tablet computer: a pilot study. Am J Hosp Palliat Care 2015;32:189–97. [DOI] [PubMed] [Google Scholar]
- [29].Vilagut G, Ferrer M, Rajmil L, et al. El Cuestionario de Salud SF-36 español: una década de experiencia y nuevos desarrollos [The Spanish version of the Short Form 36 Health Survey: a decade of experience and new developments]. Gac Sanit 2005;19:135–50. [DOI] [PubMed] [Google Scholar]
- [30].Casas Duran F, Valduvieco I, Oses G, et al. Spanish validation of Charlson index applied to prostate cancer. Clin Transl Oncol 2020;22:1187–92. [DOI] [PubMed] [Google Scholar]
- [31].Park JY, Kim MH, Han SS, et al. Clinical Research Center for End Stage Renal Disease (CRC for ESRD) Investigators. Recalibration and validation of the Charlson comorbidity index in Korean incident hemodialysis patients. PLoS One 2015;10:e0127240.doi: 10.1371/journal.pone.0127240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lee JH, Kim KI, Cho MC. Current status and therapeutic considerations of hypertension in the elderly. Korean J Intern Med 2019;34:687–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Pu C. The influence of the common cold on self-rated health: a population-based study. AIMS Public Health 2015;2:247–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hall KD, Guo J. Obesity energetics: body weight regulation and the effects of diet composition. Gastroenterology 2017;152:1718–27.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Puciato D, Borysiuk Z, Rozpara M. Quality of life and physical activity in an older working-age population. Clin Interv Aging 2017;12:1627–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Vagetti GC, Barbosa Filho VC, Moreira NB, et al. Association between physical activity and quality of life in the elderly: a systematic review, 2000-2012. Braz J Psychiatry 2014;36:76–88. [DOI] [PubMed] [Google Scholar]
- [37].Hallit S, Hajj A, Sacre H, et al. Impact of Sleep Disorders and Other Factors on the Quality of Life in General Population: A Cross-Sectional Study. J Nerv Ment Dis 2019;207:333–9. [DOI] [PubMed] [Google Scholar]


