Abstract
Background
Microvascular dysfunction, serum cytokines and chemokines may play important roles in pathophysiology of coronavirus disease 2019 (COVID-19), especially in severe cases.
Methods
Patients with COVID-19 underwent non-invasive evaluation of systemic endothelium-dependent microvascular reactivity - using laser Doppler perfusion monitoring in the skin of the forearm - coupled to local thermal hyperemia. Maximal microvascular vasodilatation (44 °C thermal plateau phase) was used as endpoint. A multiplex biometric immunoassay was used to assess a panel of 48 serum cytokines and chemokines. Severe COVID-19 (S-COVID) was defined according to WHO criteria, while all other cases of COVID-19 were considered mild to moderate (M-COVID). A group of healthy individuals who tested negative for SARS-CoV-2 served as a control group and was also evaluated with LDPM.
Results
Thirty-two patients with COVID-19 (25% S-COVID) and 14 controls were included. Basal microvascular flow was similar between M-COVID and controls (P = 0.69) but was higher in S-COVID than in controls (P = 0.005) and M-COVID patients (P = 0.01). The peak microvascular vasodilator response was markedly decreased in both patient groups (M-COVID, P = 0.001; S-COVID, P < 0.0001) compared to the healthy group. The percent increases in microvascular flow were markedly reduced in both patient groups (M-COVID, P < 0.0001; S-COVID, P < 0.0001) compared to controls. Patients with S-COVID had markedly higher concentrations of dissimilar proinflammatory cytokines and chemokines, compared to patients with M-COVID.
Conclusions
In patients with COVID-19, especially with S-COVID, endothelium-dependent microvascular vasodilator responses are reduced, while serum cytokines and chemokines involved in the regulation of vascular function and inflammation are increased.
Abbreviations: β-NGF, nerve growth factor; CCL2/MCP-1, monocyte chemoattractant protein-1; CCL3/MIP-1α, human macrophage inflammatory protein-1- α; CCL4/MIP-1β, macrophage inflammatory protein-1β; CCL5/RANTES, regulated upon activation normal Tcell expressed and secreted; CCL7/MCP-3, monocyte-specific chemokine-3; CCL247CTACK, cutaneous T cell-attracting chemokine; COVID-19, coronavirus disease 2019; CXCL10/IP-10, IFN-γ-inducible protein-10; LDPM, laser Doppler perfusion monitoring; LTH, local thermal hyperemia; FGF basic, basic fibroblast growth factor; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-monocyte colony-stimulating factor; GRO-α, growth-related oncogene-α; IFN, interferon; IL, interleukins; IL-1Ra, IL-1 receptor antagonist; IL-2Rα, soluble IL-2 receptor α; IL-12p40, IL-12 subunit p40; IL-12p40, IL-12 subunit p70; LIF, leukemia inhibitory factor; M-COVID, mild to moderate COVID-19; M-CSF, macrophage colony-stimulating factor; Mers-CoV, middle east respiratory syndrome coronavirus; MIF, migration inhibitory factor; MIG, monokine induced by interferon-γ; PDGF-BB, platelet-derived growth factor; RT-PCR, reverse transcription polymerase chain reaction; S-COVID, severe COVID-19; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SCF, stem cell factor; SCGF- β, steam cell growth factor-β; SDF-1α, stromal cell-derived factor-1α; TNF, tumor necrosis factor; TRAIL, TNF-related apoptosis inducing ligand; VEGF, vascular endothelial growth factor
Keywords: Laser Doppler perfusion monitoring, COVID-19, Endothelial dysfunction, Proinflammatory cytokines
Highlights
-
•
During acute COVID-19, endothelium-dependent microvascular vasodilator responses are reduced.
-
•
Impaired systemic microvascular vasodilation is more evident in severe vs mild-moderate COVID-19.
-
•
Increased levels of cytokines and chemokines are associated with COVID-19 severity.
1. Introduction
Coronavirus disease 2019, or COVID-19, is an acute viral illness caused by the Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV2) (Meyerowitz et al., 2020). It has affected all regions of the world, and as of 23rd November 2020, there have been 58,425,681 confirmed cases and 1,385,218 deaths, reported to the World Health Organization (WHO, 2020). It is transmitted by respiratory droplets, with an R0 of 2–3, and has a mean incubation time of five days (Meyerowitz et al., 2020). Most cases (80%), when symptomatic, present with fever and upper respiratory and gastrointestinal tract symptoms (Casas-Rojo et al., 2020; Richardson et al., 2020; Wu and McGoogan, 2020), many of which with olfactory and gustatory dysfunctions (Passali and Bentivoglio, 2020). Most importantly, around 15% of patients present a severe form of disease, that evolves over 7 to 10 days with dyspnea, a respiratory rate of over 30/min, blood oxygen saturation less than 93%, partial pressure of arterial oxygen to inspired oxygen fraction ratio < 300, and/or lung infiltrates occupying over 50% within 24 to 48 h; around 5% become critically ill, with respiratory failure, septic shock, and/or multiple organ dysfunction (Grasselli et al., 2020; Richardson et al., 2020; Wu and McGoogan, 2020). This imposes a heavy burden on intensive care beds, even in high-income countries, as recognized early on (Grasselli et al., 2020).
Two main features are noticed in the severe form: intense systemic inflammation and hypercoagulability (Connors and Levy, 2020). The expression “cytokine storm” has been proposed to depict this clinical situation (Mehta et al., 2020; Ruan et al., 2020). A multicenter study in 150 severe COVID-19 cases showed that high levels of ferritin and IL-6 were associated to death (Ruan et al., 2020). High C-reactive protein levels and d-dimer were also related to disease severity (Mehta et al., 2020).
Therapeutic measures to address the systemic inflammatory syndrome depicted have been studied. So far, there is an established role for steroids. They have been used with success in the severe forms of pulmonary disease, with a significant impact on decreasing the need for invasive ventilation and death, and rapid improvement in PaO2/FiO2 and a drop in CRP levels (Salton et al., 2020). The benefit of using steroids has been proved by a randomized trial that was conducted with a total of 2104 patients given dexamethasone 6 mg once per day for ten days and compared with 4321 patients randomized to usual care alone (Horby et al., 2020). Among the patients who received usual care alone, 28-day mortality was highest in those who required ventilation (41%), intermediate in those patients who required oxygen only (25%), and lowest among those who did not require any respiratory intervention (13%). Dexamethasone reduced deaths by one-third in ventilated patients and by one fifth in other patients receiving oxygen only. However, there was no benefit among those patients who did not require respiratory support (Horby et al., 2020).
Tocilizumab, a humanized monoclonal antibody that binds human interleukin-6 receptors, has a less clear role in severe COVID-19, as results from randomized trials reported to have failed to show mortality benefit at 28 or 30 days (Parr, 2020).
Since the beginning of the pandemic, a large number of studies have attempted to investigate the pathophysiology, clinical course and treatment options of COVID-19 (Aguilar et al., 2020; Petrilli et al., 2020; Psotka et al., 2020; Stratton et al., 2020). Understanding the pathophysiology of COVID-19 is crucial for scientific knowledge, the ability to identify clinical correlations, and the development of therapeutic strategies. In that context, microvascular dysfunction has been shown to play a key role in the pathophysiology of COVID-19 (Damiani et al., 2020; De Lorenzo et al., 2020a; Pons et al., 2020; Sardu et al., 2020). In fact, it has been suggested that vascular endothelial cells are a common link between the invasion of the body by SARS-CoV-2 and pre-existing cardiometabolic diseases (De Lorenzo et al., 2020b; Tibirica and De Lorenzo, 2020a, Tibirica and De Lorenzo, 2020b). In addition, patients diagnosed with moderate or severe COVID-19 present increased levels of proinflammatory cytokines, particularly interleukin-6 and tumor necrosis factor-α (G. Chen et al., 2020). Cytokine release syndrome (“cytokine storm”), which is characterized by a massive increase in the levels of proinflammatory cytokines, is known to be the major cause of morbidity and mortality in patients infected by SARS-CoV-2 (G. Chen et al., 2020).
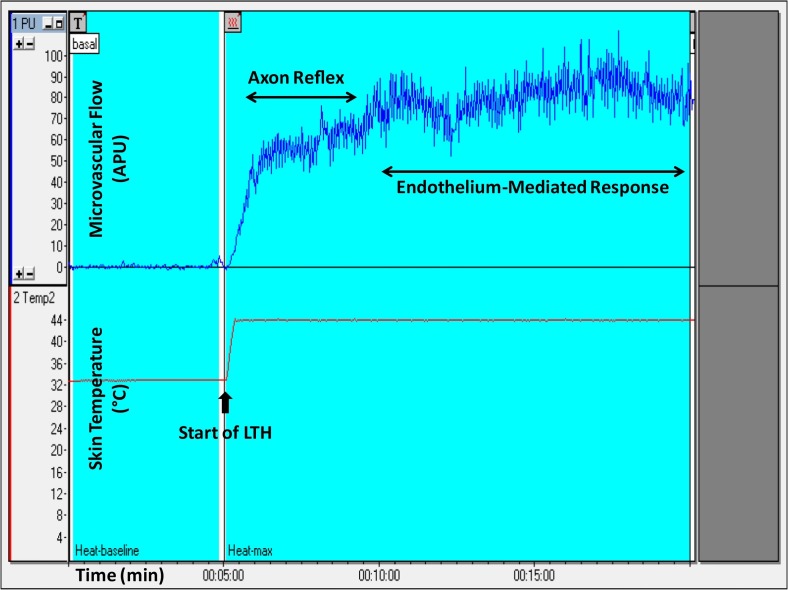
Clinical studies on microcirculatory physiology have been performed for a long time, in the context of several conditions (Roustit and Cracowski, 2012), and using different methods (Cracowski and Roustit, 2016). Of note, the cutaneous microcirculation is an accessible and representative vascular bed that can be used for the evaluation of systemic microcirculatory flow and reactivity (Holowatz et al., 2008). Laser Doppler perfusion monitoring (LDPM) is a noninvasive method for the evaluation of systemic microvascular endothelial function, and LDPM has already proven useful in many clinical conditions (Turner et al., 2008), including cardiovascular and metabolic diseases (de Moraes et al., 2016; Gomes et al., 2008; Kaiser et al., 2013), rheumatologic diseases (Ruaro et al., 2017; Weibel et al., 2007) and circulatory shock (either septic or cardiogenic) (Mongkolpun et al., 2020; Orbegozo et al., 2018). Most published data concerning LDPM have validated the site of the skin of the forearm for the measurements, which is more accessible and more representative of general skin microvascular function (Cracowski and Roustit, 2016). Systemic microvascular reactivity can be evaluated using LDPM combined with cutaneous local thermal hyperemia (LTH), as the vasodilatory response in the skin due to LTH represents, fundamentally, endothelium-dependent microvascular reactivity (Roustit and Cracowski, 2012; Ugenti et al., 2018). In fact, LTH induces a biphasic curve of cutaneous microvascular vasodilation; the initial peak (2–5 min) represents the local sensory nerve axon reflex (Roustit and Cracowski, 2013), while the second peak (15 min) characterizes the release of endothelial vasodilators, predominantly nitric oxide (Roustit and Cracowski, 2013). This methodology has already been used in the description of endothelial dysfunction and altered microvascular reactivity in different cardiovascular and metabolic diseases, including arterial hypertension (Rossi et al., 2008; Ullyot, 1990; Virdis and Taddei, 2011) type 1 diabetes (Sorelli et al., 2019), hypercholesterolemia (Holowatz and Kenney, 2011; Holowatz et al., 2011) polycystic ovary syndrome and metabolic syndrome (Ketel et al., 2008). Furthermore, LTH has been used as a clinical surrogate marker in various diseases, such as Raynaud's phenomenon and systemic sclerosis (Boignard et al., 2005; Roustit et al., 2008). In the present study, maximal endothelium-dependent microvascular vasodilatation (44 °C thermal plateau phase) was measured during the last 5 min of the second peak of microvascular flow resulting from LTH (a representative example of the recordings is presented in Fig. 1 ).
Fig. 1.
Representative recording of skin microvascular flow using laser Doppler perfusion monitoring coupled with local thermal hyperemia (LTH) of a healthy volunteer.
APU, arbitrary perfusion units.
Given that the presence and intensity of systemic microvascular changes during the acute phase of COVID-19 may be related to disease progression and prognosis, the evaluation of microvascular reactivity in COVID-19 patients may offer relevant information. Thus, we used LDPM, which provides a noninvasive, simple and repeatable evaluation of tissue perfusion (Tibirica and De Lorenzo, 2020a), combined with LTH, to compare the systemic microvascular flow and reactivity in patients with mild-to-moderate or severe COVID-19 with that in age-matched healthy volunteers who tested negative for SARS-CoV-2. We also evaluated the plasma levels of different cytokines and chemokines with pro- or anti-inflammatory effects or with biological effects on vascular function, as these molecules might be involved in the pathophysiology of endothelial dysfunction in the context of acute COVID-19.
2. Methods
Patients with COVID-19 who were admitted to the National Institute of Cardiology, Ministry of Health, Rio de Janeiro, Brazil, were included after signing an informed consent form. A responsible family member signed the informed consent form when the patients were unable to sign it themselves. All the patients had underlying cardiac disease. The study was approved by the Institutional Review Board and was registered and made public at ClinicalTrials.gov (NCT4406545).
The patients had SARS-CoV-2, which was detected by RT-PCR analysis of nasopharyngeal swabs, and met the criteria for hospitalization either due to their underlying condition or due to COVID-19 (Berlin et al., 2020; Wiersinga et al., 2020). The demographic, clinical and laboratory data were recorded. The laboratory data included in this study were obtained within 24 h of the assessment of microvascular reactivity, and the serum cytokine levels were evaluated on the same day when LDPM was performed.
Severe COVID-19 (S-COVID) was defined according to the WHO criteria, while all the other cases of COVID-19 were considered mild to moderate (M-COVID) (https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf). Due to the very distinct characteristics and management (especially the need for vasoactive drugs), the groups were analyzed separately: patients without severe disease (M-COVID) and patients with severe disease (S-COVID).
A group of healthy volunteers (without chronic diseases or cardiac risk factors) was recruited from hospital staff members who tested negative for SARS-CoV-2. This group was also evaluated with LDPM and served as a control group. This group did not undergo cytokine assessment.
2.1. Evaluation of microvascular flow and reactivity
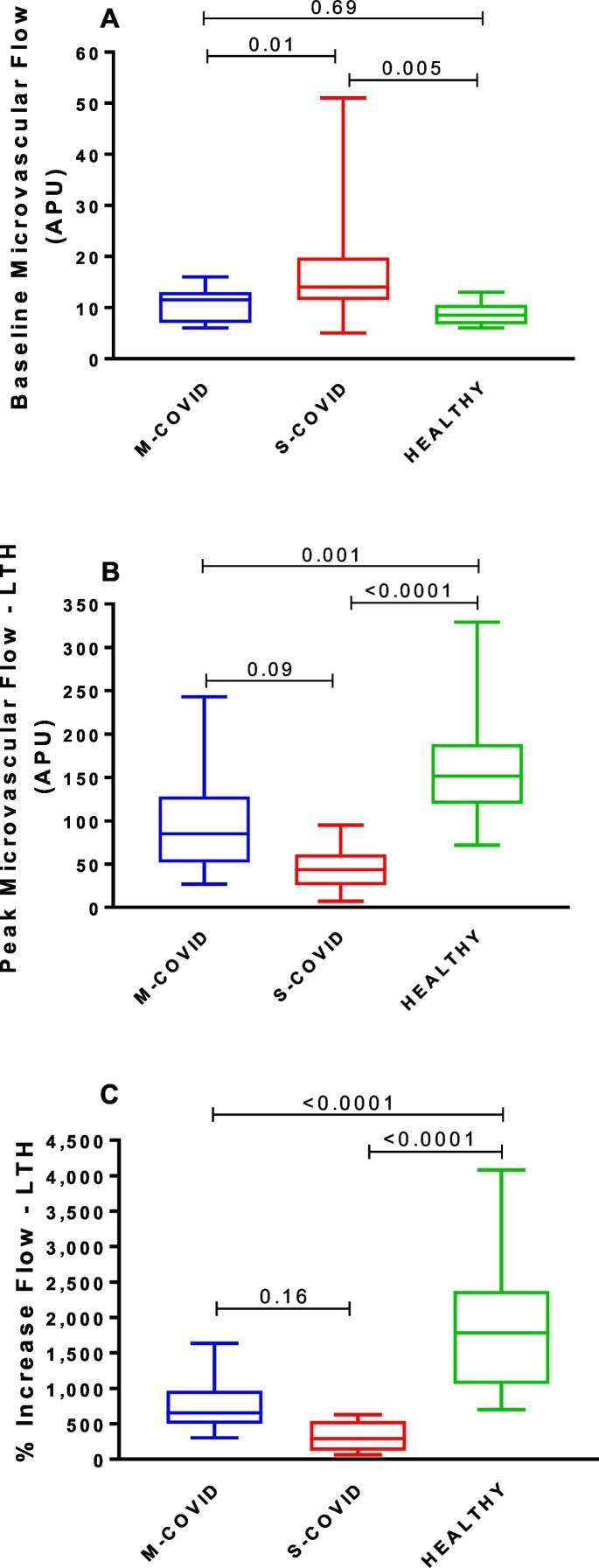
The evaluation of the microvascular flow and reactivity was performed within one week after admission, during the acute phase of disease, using a single-point laser Doppler perfusion monitoring (LDPM) system (Periflux 5001, Perimed, Järfälla, Sweden) to noninvasively measure systemic microvascular perfusion changes (in arbitrary perfusion units [APU = 10 mV]), as previously described (Cracowski and Roustit, 2016; Salgado et al., 2014), using heating laser probes (PF 457, Perimed). The probes were attached to the skin with adhesive tapes, thus limiting movement artifacts. Moreover, patients were asked not to move during the recording period. After measuring the resting microvascular flow on the skin of the forearm for 5 min, the maximal microvascular vasodilatation was assessed using prolonged (20 min) local heating of the laser probe to 44 °C (local thermal hyperemia, LTH, Fig. 1), as previously described in detail (Salgado et al., 2014; Ugenti et al., 2018). Maximal vasodilatation (44 °C thermal plateau phase) corresponds to the mean microvascular flow during the last 5 min of the second peak. Data were digitized and stored on a computer and analyzed off-line with signal processing software (PeriSoft, Perimed, Järfälla, Sweden). We also compared the individual percentage increase of endothelium-dependent microvascular conductance (Fig. 2C), to normalize the results to baseline values of microvascular flow, which is measure in arbitrary perfusion units.
Fig. 2.
Effects of local thermal hyperemia (LTH) on cutaneous microvascular flow and reactivity in patients with mild-to-moderate (M-COVID) or severe (S-COVID) COVID-19 and in COVID-19-negative age-matched healthy volunteers: (A) Baseline cutaneous microvascular flow; B) Maximum microvascular flow during LTH and C) percent increase in microvascular flow induced by LTH (from baseline to peak microvascular flow).
The values are expressed as box and whisker plots, where the centerline denotes the median value, the box contains the 25th to 75th percentiles of the dataset and the whiskers indicate the maximum and minimum values. The results were analyzed using one-way ANOVA.
APU, arbitrary perfusion units.
2.2. Multiplex immunoassay
Blood samples were collected from a peripheral vein and stored on ice. Plasma was obtained by centrifugation at 800g for 15 min at 4 °C, aliquoted, and stored at −70 °C until the day of analysis. A multiplex biometric immunoassay, using fluorescently dyed microspheres conjugated to monoclonal antibodies specific for a target protein, was used to measure 48 cytokine and chemokine cell signaling molecules according to the manufacturer's instructions (Bio-Plex Human Cytokine Assay; Bio-Rad Inc., Hercules, CA, USA). The cytokines measured were interleukins, including IL-1α, IL-1β, IL-1 receptor antagonist (IL-1Ra), IL-2, soluble IL-2 receptor α (IL-2Rα), IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12 subunit p40(IL-12p40), IL-12 subunit p70 (IL-12p70), IL-13, IL-15, IL-16, IL-17A, and IL-18; interferons, including IFN-α2 and IFN-γ; tumor necrosis factors (TNF-α, TNF-β, and TNF-related apoptosis inducing ligand [TRAIL]); growth factors, including stem cell factor (SCF), basic fibroblast growth factor (FGF basic), nerve growth factor (β-NGF), hepatocyte growth factor (HGF), leukemia inhibitory factor (LIF), platelet-derived growth factor (PDGF-BB), vascular endothelial growth factor (VEGF), steam cell growth factor-β (SCGF- β), granulocyte colony-stimulating factor (G-CSF), macrophage colony-stimulating factor (M-CSF) and granulocyte-monocyte colony-stimulating factor (GM-CSF); and finally chemokines, including cutaneous T cell-attracting chemokine (CCL247CTACK), eotaxin, growth-related oncogene-α (GRO-α), IFN-γ-inducible protein-10 (CXCL10/IP-10), monocyte chemoattractant protein-1 (CCL2/MCP-1), monocyte-specific chemokine-3 (CCL7/MCP-3), migration inhibitory factor (MIF), monokine induced by interferon-γ (MIG), macrophage inflammatory protein (CCL3/MIP-1α and CCL4/MIP-1β), regulated upon activation normal Tcell expressed and secreted (CCL5/RANTES), and stromal cell-derived factor-1α (SDF-1α). The cytokine levels were determined using a multiplex array reader from Luminex™ Instrumentation System (Bio-Plex Workstation from Bio-Rad Laboratories, Hercules, California, USA). The analyte concentrations were calculated using software provided by the manufacturer (Bio-Plex Manager Software).
2.3. Statistical analysis
The results are presented as the mean ± SD or median (25th–75th percentiles) for the parametric or nonparametric parameters, respectively, according to the Shapiro-Wilk normality test. The statistical analyses were performed using one-way ANOVA followed by Tukey's multiple comparisons test. The cytokine and chemokine data were analyzed using the two-tailed unpaired Mann-Whitney test. Contingency tables were analyzed using chi-square tests. P values < 0.05 were considered statistically significant. All the statistical analyses were performed using Prism, version 7.0 (GraphPad Software Inc. La Jolla, CA, USA).
3. Results
3.1. Clinical and demographic characteristics
Thirty-two COVID-19 patients and 14 healthy volunteers were included. Table 1 depicts the demographic and clinical characteristics of these individuals. Twenty-four (75%) patients had mild-to-moderate disease and were managed in regular hospital beds, while 8 (25%) required admission to the ICU for mechanical ventilation and/or hemodynamic support. The mean age of the patients with M-COVID was 58.2 ± 14.1 years, and that of the patients with S-COVID was 65.6 ± 6.5 years; in these groups, 54% and 100% of the patients were male, respectively.
Table 1.
The clinical characteristics of healthy controls and mild-to-moderate COVID-19 (M-COVID) or severe COVID-19 (S-COVID) patients.
| Parameter | M-COVID (n = 24) | S-COVID (n = 8) | HEALTHY (n = 14) | P-value |
|---|---|---|---|---|
| Age (years) | 58.2 ± 14.1 | 65.6 ± 6.5 | 56.3 ± 9.6 | 0.20 |
| Male n (%) | 13 (54) | 8 (100) | 6 (43) | 0.03 |
| SAP (mmHg) | 115 ± 14 | 131 ± 28 | 133 ± 22 | 0.01 |
| DAP (mmHg) | 69 ± 10 | 68 ± 15 | 80 ± 9 | 0.01 |
| MAP (mmHg) | 86 ± 11 | 89 ± 18 | 98 ± 11 | 0.02 |
| BMI (kg/m2) | 28.3 ± 5.5 | 29.5 ± 2.1 | N/D | 0.55 |
| Heart rate (bpm) | 73 ± 13 | 93 ± 24 | N/D | 0.003 |
| SaO2 (%) | 96.4 ± 2.3 | 98.5 ± 1.8 | N/D | 0.02 |
| Hypertensionn (%) | 18 (75) | 8 (100) | N/A | 0.30 |
| Diabetes n (%) | 12 (50) | 5 (65.5) | N/A | 0.73 |
| Dyslipidemia n (%) | 7 (29.2) | 0 (0) | N/A | 0.15 |
| Smoking n (%) | 7 (29.2) | 2 (25) | N/A | >0.99 |
| Coronary artery disease n (%) | 17 (70.8) | 6 (75) | N/A | >0.99 |
| Valvular heart disease n (%) | 5 (20.8) | 0 (0) | N/A | 0.30 |
| Usual medications | ||||
| Angiotensin receptor blockers/ACE inhibitors n (%) |
14 (58.3) | 0 (0) | N/A | 0.004 |
| Beta-blockers n (%) | 21 (87.5) | 1 (12.5) | N/A | 0.003 |
| Calcium channel blockers n (%) | 5 (20.8) | 1 (12.5) | N/A | >0.99 |
| Direct vasodilators n (%) | 2 (8.3) | 1 (12.5) | N/A | >0.99 |
| Nitrates n (%) | 2 (8.3) | 0 (0) | N/A | >0.99 |
| Diuretics n (%) | 16 (66.7) | 2 (25) | N/A | 0.09 |
| Statins n (%) | 17 (70.8) | 1 (12.5) | N/A | 0.01 |
| Oral antidiabetic agents n (%) | 2 (8.3) | 0 (0) | N/A | >0.99 |
| Insulin n (%) | 10 (41.7) | 7 (87.5) | N/A | 0.04 |
| Antiplatelet agents n (%) | 20 (83.3) | 2 (25.0) | N/A | 0.005 |
| Oral anticoagulant agents n (%) | 1 (4.2) | 0 (0) | N/A | >0.99 |
| Inpatient management | ||||
| Antibiotics n (%) | 6 (25) | 5 (62.5) | N/A | 0.09 |
| Oseltamivir n (%) | 2 (8.3) | 1 (12.5) | N/A | >0.99 |
| Hydroxychloroquine n (%) | 0 (0) | 0 (0) | N/A | >0.99 |
| Vasoactive amines n (%) | 0 (0) | 5 (62.5) | N/A | 0.0003 |
| Heparin n (%) | 20 (83.3) | 7 (87.5) | N/A | >0.99 |
| Supplementary oxygen (nasal cannula) n (%) |
5 (20.8) | 0 (0) | N/A | 0.30 |
| Mechanical ventilation n (%) | 0 (0) | 7 (87.5) | N/A | <0.0001 |
| Laboratory data | ||||
| Hematocrit (%) | 36.7 (33.6–40.1) | 24.2 (21.8–30.8) | N/D | <0.0001 |
| Hemoglobin (g/dL) | 11.9 ± 2.1 | 8.4 ± 1.7 | N/D | 0.0001 |
| Leukocytes (mm3) | 7687 ± 3267 | 11,774 ± 3237 | N/D | 0.004 |
| Platelets (mm3) | 238,297 ± 99,642 | 218,500 ± 107,980 | N/D | 0.64 |
| hs-CRP (mg/L) | 4.2 (1.2–10.3) | 14.7 (5.6–27.6) | N/D | 0.02 |
| Glycemia (mg/dL) | 135 (125–175) | 168 (133–187) | N/D | 0.37 |
| Urea (mg/dL) | 44 (29.5–77.5) | 117.5 (43.2–240.8) | N/D | 0.05 |
| Creatinine (mg/dL) | 1.05 (0.86–1.61) | 1.99 (1.01–3.26) | N/D | 0.10 |
SAP, systolic arterial pressure; DAP, diastolic arterial pressure; MAP, mean arterial pressure; ACE, angiotensin-converting enzyme; BMI, body mass index;SaO2, oxygen saturation; hs-CRP, high sensitivity C-reactive protein; N/D, not determined; N/A, not applicable.
The results are presented as the mean ± SD or the median (25th–75th percentile) for values that follow or do not follow a Gaussian distribution, respectively (Shapiro-Wilk normality test).
P-values were estimated using two-tailed unpaired Student's t-tests (comparisons of two groups for parameters with Gaussian distribution), two-tailed unpaired Mann-Whitney tests (comparisons of two groups for parameters with non-Gaussian distribution), or chi-square (Fisher's exact test), for categorical parameters. The parameters evaluated in the three groups of individuals (M-COVID, S-COVID and HEALTHY) were analyzed using one-way ANOVA.
3.2. Microvascular function evaluation
Evaluation of the microvascular flow and reactivity (Fig. 2) showed that the basal flow was similar between the M-COVID patients and healthy volunteers (P = 0.69) but was higher in the S-COVID patients than in the healthy volunteers (P = 0.005) and M-COVID patients (P = 0.01; Fig. 2A). The peak microvascular vasodilator response induced by LTH was markedly decreased in both the patient groups (M-COVID, P = 0.001; S-COVID, P < 0.0001) compared to the healthy volunteer group (Fig. 2B). The flow during LTH tended to be lower in the S-COVID patients than in the M-COVID patients (p = 0.09). Finally, the percent increases in the microvascular flow, from baseline to peak flow during LTH, were also markedly decreased in both the patient groups (M-COVID, P < 0.0001; S-COVID, P < 0.0001) compared to the healthy volunteer group (Fig. 2C).
3.3. Serum cytokine and chemokine levels
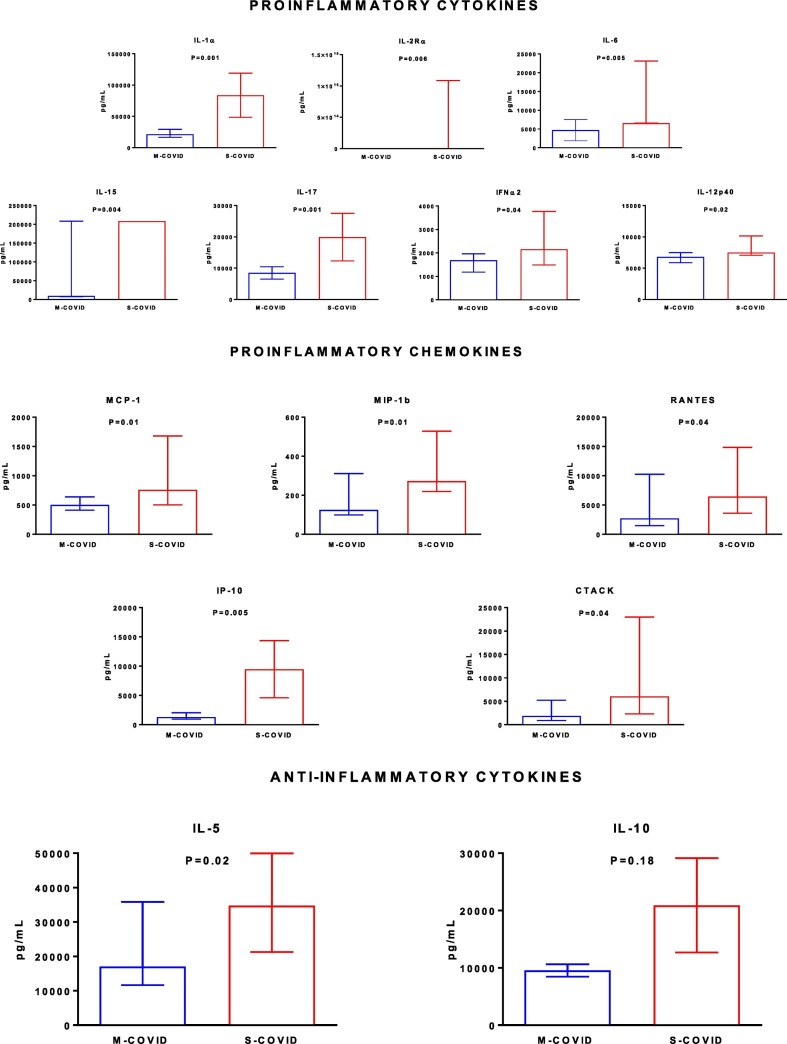
Of the 48 cytokines and chemokines tested, several displayed significantly different serum concentrations between the patients with M-COVID and the patients with S-COVID. Regarding proinflammatory cytokines, the patients with S-COVID had higher serum concentrations of IL-1α, IL-2Rα, IL-6, IL-15, IL-17, IFNα2 and IL-2p40 (Fig. 3 ).
Fig. 3.
Plasma concentrations of proinflammatory cytokines, proinflammatory chemokines and anti-inflammatory cytokines in patients with mild-to-moderate (M-COVID) or severe (S-COVID) COVID-19.
The values are expressed as the medians (25th to 75th percentiles) and were analyzed using the two-tailed unpaired Mann-Whitney test.
The patients with S-COVID also had higher serum concentrations of proinflammatory chemokines, including MCP-1, MIP1β, RANTES, and IP-10 (Fig. 3). Finally, the anti-inflammatory cytokine IL-5 was also increased in the patients with S-COVID compared to the patients with M-COVID (Fig. 3).
4. Discussion
The main findings of this study are as follows: (i) endothelium-dependent microvascular vasodilator responses evaluated using LDPM combined with LTH are reduced in patients with COVID-19, especially in those with severe COVID-19, and (ii) the levels of serum cytokines and chemokines involved in the regulation of vascular function and inflammation are increased in patients with severe COVID-19 compared to those with nonsevere disease.
In our study, the baseline microvascular flow was higher in the patients with S-COVID than in the healthy volunteers. The marked inflammatory response observed in patients with S-COVID, demonstrated by the higher plasma concentrations of cytokines and chemokines, compared to M-COVID patients, most likely induced systemic vasodilation that resulted in increased microvascular conductance. Moreover, in the severely ill patients, systemic vasodilation and decreased tissue perfusion, which are associated with cellular and metabolic abnormalities, require the use of vasopressors to improve tissue perfusion and microvascular alterations by restoring the perfusion pressure in vital organs (De Backer and Foulon, 2019). In our study sample, five out of eight S-COVID patients were being treated with vasopressor agents, which could have increased the perfusion pressure and, consequently, the baseline systemic microvascular flow. Of note, the mean arterial pressure of the S-COVID patients was maintained in the normal range (89 ± 18 mmHg) during the study procedures.
In this series of patients, during the acute phase of COVID-19, the systemic endothelium-dependent microvascular reactivity in both the severe and mild-to-moderate patients was impaired compared with that in the sex- and age-matched healthy volunteers. In fact, both maximum microvascular vasodilation and increase in microvascular conductance induced by LTH, compared to baseline values, were markedly impaired in patients with S-COVID, compared with healthy volunteers. Moreover, the impairment was more pronounced in the patients with S-COVID and occurred in parallel with the increased plasma levels of proinflammatory cytokines and chemokines in these patients.
In our study, mild to moderate COVID-19 patients (M-COVID) were admitted to a clinical ward and received less intensive support than those with severe COVID-19 (S-COVID), who were all in the Intensive Care Unit (ICU) and 7 out of 8 on mechanical ventilation. Therefore, the SaO2 was (marginally, 98 vs. 96%) higher in S-COVID likely due to ventilation with high oxygen concentrations, versus only oxygen supplementation through facial mask or nasal cannula in M-COVID. Regarding medications, we should note that most patients with S-COVID, in the ICU, were on vasopressor medications, and due to hemodynamic instability had their blood pressure-lowering medications withdrawn, contributing to a lower number of medications than in patients with M-COVID.
Importantly, our results demonstrated that increased levels of proinflammatory cytokines, chemokines, and anti-inflammatory interleukins are associated with COVID-19 severity. Consistent with our findings, a recent experimental study of SARS-CoV-2-infected mice and MERS-CoV-infected human microvascular endothelial cells demonstrated that the expression of the genes encoding the interleukins IL-1α and IL-6, the chemokines MCP-1, RANTES, and IP-10, and IFN-α2 was significantly increased within 24 h of infection, and this increased expression was related to the high infiltration of T cells, NK cells and monocytes (Yao et al., 2020).
Regarding individual cytokines, interleukins 1α, 2Rα, 6, 15, 17, 12p40, interferon α2, IP 10, MCP1, MIP 1b, RANTES and CTACK, all with proinflammatory activities, were increased in patients with S-COVID compared to M-COVID, as well as the anti-inflammatory cytokines IL5 and IL10 (the latter, non significantly).
IL-1α is closely linked to the innate immune response and assists in host defense against infection (Dinarello, 2009). Soluble IL-2Rα is found at high levels in the circulation of healthy individuals but is further increased in patients with infection, inflammation and autoimmune diseases (Damoiseaux, 2020). Soluble IL-2Rα and IL-6 are significantly correlated with the severity of lung injury and the ratio of arterial oxygen partial pressure to fractional inspired oxygen (PaO2/FiO2) in COVID-19 patients with pneumonia. In addition, IL-6 is considered an independent predictor of adverse prognosis in COVID-19 patients (N. Chen et al., 2020; Ruan et al., 2020; Zhou et al., 2020).
IL-15 is involved in the early, innate immune defense against viral infections in humans. Macrophage-derived IL-15 contributes with other monokines (especially IL-12) to the proinflammatory cascade leading to innate immune IFN-γ production (Fehniger and Caligiuri, 2001).
Interferons are essential in the response to viral infections, and the IFN-α response in SARS-CoV has been demonstrated in ferrets (Danesh et al., 2011). Recently, the administration of IFN-α has been tested to negativize viral RNA in COVID-19 patients (Idelsis et al., 2020).
MCP-1 is a predictor of the outcome of severe sepsis and is associated with the activated protein C pathway and the genes it induces (N. Chen et al., 2020). Our results are consistent with a recent study that showed that chemokines, such as IP-10 and MCP-1, are upregulated in monocytes/macrophages infected with SARS-CoV and are highly involved in the clinical progression of MERS (Yao et al., 2020). Additionally, IP-10 and MCP-1 were found to be elevated in COVID-19 patients who needed intensive care treatment (Liu et al., 2020; Wang et al., 2020).
MIP-1β has been shown to be induced by dengue virus infection (Spain-Santana et al., 2001). MIP-1β is produced by human monocytes and dendritic cells upon different stimuli and is a chemoattractant for NK cells, recruiting them to inflammatory sites. NK cells have been associated with mild dengue (Azeredo et al., 2006). In the study by Bozza et al. (2008), a correlation between MIP-1β plasma levels and NK cells was observed, suggesting their role in dengue protective mechanisms. On the contrary, in our study, MIP-1β was higher in patients with severe COVID-19; larger studies may elucidate if there is a different pattern of MIP-1β action in COVID-19 compared to dengue infection.
IL-12 and IL-17A were shown to upregulate ACE2 mRNA expression in the airway epithelial cells of patients with severe COVID-19 and chronic obstructive pulmonary disease (Ruan et al., 2020). Some of the biological activities of IL-12 include enhancement of cytotoxic T cells, generation and activation of lymphokine-activated killer cells, increase in natural killer (NK) cell cytotoxicity, induction of activated T cell and NK cell proliferation, and induction of IFN-γ production by T and NK cells. IL-12 is secreted by activated B cells, macrophages, and other antigen-presenting cells, and its production is inhibited by IL-4 and IL-10. Therefore, IL-12 plays an important role in cell-mediated inflammation and contributes to the regulation of immunoglobulin production (Zundler and Neurath, 2015). The increased levels of IL-12 in S-COVID patients may reflect the ongoing immune response to SARS-CoV-2 infection.
IL-17, another proinflammatory cytokine that is expressed at higher levels in S-COVID patients, is a product of activated T lymphocytes, and its biological activities include stimulation of IL-6 and IL-8 production. IL-17 is highly produced in patients with chronic inflammatory diseases but is also involved in the pathophysiology of the cardiovascular complications associated with autoimmune and inflammatory diseases (Maione, 2016). Interestingly, elevated IL-17A levels correlate with vascular dysfunction in rheumatoid arthritis patients (Marder et al., 2011). Thus, the elevation of IL-17 might be one of the causative factors of the microvascular dysfunction observed in our study. Finally, IL-17 has been shown to increase platelet activation and arterial thrombus formation (Maione et al., 2011), which might help to explain the thrombotic phenomena found in COVID-19 patients (Raucci et al., 2020).
RANTES, also known as chemokine ligand CCL5, is involved in vascular dysfunction by modulating perivascular inflammation. RANTES is an important chemoattractant for inflammatory cells that seems to be involved in the pathogenesis of atherosclerosis in humans and in experimental mouse models (Maione, 2016; Marder et al., 2011). RANTES can be produced by resident cells in vessels and atherosclerotic plaques, and in in vitro experiments, its expression is increased by angiotensin II in the arteriolar and venular endothelium (Maione et al., 2011). Experimental findings also suggest that RANTES is involved in obliterative changes in the pulmonary arteries of patients with pulmonary arterial hypertension (Raucci et al., 2020). In addition, studies have shown that RANTES inhibitors significantly decreased viremia in SARS-CoV-2 patients (Raucci et al., 2020).
CTACK is constitutively expressed in normal skin and is upregulated in wound sites. This chemokine is elevated in the cerebrospinal fluid samples of HIV-1-positive patients and is correlated with nonneurological disorders (Kopf et al., 1996). However, our study is the first to demonstrate the increase in the CTACK levels in patients with COVID-19. The significance of this finding has yet to be determined.
The complexity of the cytokine/chemokine responses in COVID-19 patients is noteworthy. Huang et al. (2020) showed that patients infected with SARS-CoV-2 had high levels of IL1β, IFNγ, IP10, and MCP1, probably leading to activated T helper 1 (Th1) cell responses, and patients requiring ICU admission had higher concentrations of G-CSF, IP10, MCP1, MIP1α, and TNFα than those not requiring ICU admission, suggesting that the cytokine storm was associated with disease severity. However, COVID-19 patients also exhibited increased secretion of T helper 2 (Th2) cytokines (e.g., IL-4 and IL-10), which suppress inflammation. This observation is consistent with our results, as anti-inflammatory cytokines such as IL-5, which has a very narrow set of cellular targets, such as human eosinophils, basophils and a subset of mast cells, and, thus far, has not been described in COVID-19 patients (Kopf et al., 1996) and IL-10, which play a central role in limiting the host immune response to pathogens, thereby limiting damage to the host (Iyer and Cheng, 2012), were increased (although not significantly in the case of IL-10) in patients with S-COVID. Further studies may elucidate the interplay between proinflammatory/anti-inflammatory mediators, as well as their vascular effects, in COVID-19 patients.
Limitations to this study must be considered. The M-COVID and S-COVID groups are imbalanced for number, age and sex. This is due to the “real-world” nature of the study, which was performed with incoming patients amidst the COVID-19 pandemic; therefore, the differences reflect epidemiologic characteristics of the pandemic, such as the higher number of male patients with severe disease, as well as increased age in this subgroup.
In conclusion, endothelium-dependent microvascular vasodilator responses evaluated using LDPM combined with LTH are decreased in patients with COVID-19, especially in those with severe COVID-19. The levels of serum cytokines and chemokines involved in the regulation of vascular function and inflammation are increased in patients with severe COVID-19 compared to those with mild-to-moderate COVID.
Funding sources
This investigation was supported by grants from CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, E.T. grant # 305234/2017-0 and H.C.C.F.N grant # 4017000/2020-8), FAPERJ (Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro, E.T. grant # E-26/202.822/2018 and H.C.C.F.N grant # E26/210.181/2020).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank Marcio Marinho Gonzalez for his excellent technical assistance with laser speckle recordings and Edson Fernandes de Assis for performing the cytokine assays with the multiplex platform of the Instituto Oswaldo Cruz, Fiocruz, Rio de Janeiro. We would also like to thank Adriana Bastos Carvalho, Izabella Pereira da Silva Bezerra, Raiana Andrade Quintanilha Barbosa, TaísHanae Kasai Brunswick and Glauber Monteiro Dias, for technical assistance in the procedures of blood processing and deep-freeze storage.
References
- Aguilar, R. B., et al., 2020. Current understanding of COVID-19 clinical course and investigational treatments. Front Med (Lausanne). 7, 555301. [DOI] [PMC free article] [PubMed]
- Azeredo E.L. NK cells, displaying early activation, cytotoxicity and adhesion molecules, are associated with mild dengue disease. Clin. Exp. Immunol. 2006;143:345–356. doi: 10.1111/j.1365-2249.2006.02996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin D.A. Severe Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMcp2009575. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- Boignard A. Local hyperemia to heating is impaired in secondary Raynaud’s phenomenon. Arthritis Res. Ther. 2005;7:R1103–R1112. doi: 10.1186/ar1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozza F.A. Multiplex cytokine profile from dengue patients: MIP-1beta and IFN-gamma as predictive factors for severity. BMC Infect. Dis. 2008;8:86. doi: 10.1186/1471-2334-8-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas-Rojo J.M. Clinical characteristics of patients hospitalized with COVID-19 in Spain: results from the SEMI-COVID-19 Registry. Rev. Clin. Esp. 2020;220:480–494. doi: 10.1016/j.rce.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors J.M., Levy J.H. Thromboinflammation and the hypercoagulability of COVID-19. J. Thromb. Haemost. 2020;18:1559–1561. doi: 10.1111/jth.14849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cracowski J.L., Roustit M. Current methods to assess human cutaneous blood flow: an updated focus on laser-based-techniques. Microcirculation. 2016;23:337–344. doi: 10.1111/micc.12257. [DOI] [PubMed] [Google Scholar]
- Damiani E. Microvascular alterations in patients with SARS-COV-2 severe pneumonia. Ann. Intensive Care. 2020;10:60. doi: 10.1186/s13613-020-00680-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux J. The IL-2 - IL-2 receptor pathway in health and disease: the role of the soluble IL-2 receptor. Clin. Immunol. 2020;218:108515. doi: 10.1016/j.clim.2020.108515. [DOI] [PubMed] [Google Scholar]
- Danesh A. Early gene expression events in ferrets in response to SARS coronavirus infection versus direct interferon-alpha2b stimulation. Virology. 2011;409:102–112. doi: 10.1016/j.virol.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Backer D., Foulon P. Minimizing catecholamines and optimizing perfusion. Crit. Care. 2019;23:149. doi: 10.1186/s13054-019-2433-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lorenzo A. Systemic endothelial dysfunction: a common pathway for COVID-19, cardiovascular and metabolic diseases. Nutr. Metab. Cardiovasc. Dis. 2020;30(8):1401–1402. doi: 10.1016/j.numecd.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lorenzo A. Systemic endothelial dysfunction: a common pathway for COVID-19, cardiovascular and metabolic diseases. Nutr. Metab. Cardiovasc. Dis. 2020;30:1401–1402. doi: 10.1016/j.numecd.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Moraes R. Effects of non-supervised low intensity aerobic excise training on the microvascular endothelial function of patients with type 1 diabetes: a non-pharmacological interventional study. BMC Cardiovasc. Disord. 2016;16:23. doi: 10.1186/s12872-016-0191-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C.A. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- Fehniger T.A., Caligiuri M.A. Interleukin 15: biology and relevance to human disease. Blood. 2001;97:14–32. doi: 10.1182/blood.v97.1.14. [DOI] [PubMed] [Google Scholar]
- Gomes M.B. Evaluation of microvascular endothelial function in patients with type 1 diabetes using laser-Doppler perfusion monitoring: which method to choose? Microvasc. Res. 2008;76:132–133. doi: 10.1016/j.mvr.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Grasselli G. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020;323:1545–1546. doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- Holowatz L.A., Kenney W.L. Acute localized administration of tetrahydrobiopterin and chronic systemic atorvastatin treatment restore cutaneous microvascular function in hypercholesterolaemic humans. J. Physiol. 2011;589:4787–4797. doi: 10.1113/jphysiol.2011.212100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holowatz L.A. The human cutaneous circulation as a model of generalized microvascular function. J. Appl. Physiol. 2008;105:370–372. doi: 10.1152/japplphysiol.00858.2007. (1985) [DOI] [PubMed] [Google Scholar]
- Holowatz L.A. Oral atorvastatin therapy restores cutaneous microvascular function by decreasing arginase activity in hypercholesterolaemic humans. J. Physiol. 2011;589:2093–2103. doi: 10.1113/jphysiol.2010.203935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horby P. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2021436. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idelsis, E.-M., et al., 2020. Effect and safety of combination of interferon alpha-2b and gamma or interferon alpha-2b for negativization of SARS-CoV-2 viral RNA. Preliminary results of a randomized controlled clinical trial. medRxiv. 2020.07.29.20164251.
- Iyer S.S., Cheng G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit. Rev. Immunol. 2012;32:23–63. doi: 10.1615/critrevimmunol.v32.i1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser S.E. Antihypertensive treatment improves microvascular rarefaction and reactivity in low-risk hypertensive individuals. Microcirculation. 2013;20:703–716. doi: 10.1111/micc.12067. [DOI] [PubMed] [Google Scholar]
- Ketel I.J. Obese but not normal-weight women with polycystic ovary syndrome are characterized by metabolic and microvascular insulin resistance. J. Clin. Endocrinol. Metab. 2008;93:3365–3372. doi: 10.1210/jc.2008-0626. [DOI] [PubMed] [Google Scholar]
- Kopf M. IL-5-deficient mice have a developmental defect in CD5+ B-1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity. 1996;4:15–24. doi: 10.1016/s1074-7613(00)80294-0. [DOI] [PubMed] [Google Scholar]
- Liu K. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin. Med. J. 2020;133:1025–1031. doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maione F. Commentary: IL-17 in chronic inflammation: from discovery to targeting. Front. Pharmacol. 2016;7:250. doi: 10.3389/fphar.2016.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maione F. IL-17A increases ADP-induced platelet aggregation. Biochem. Biophys. Res. Commun. 2011;408:658–662. doi: 10.1016/j.bbrc.2011.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder W. Interleukin 17 as a novel predictor of vascular function in rheumatoid arthritis. Ann. Rheum. Dis. 2011;70:1550–1555. doi: 10.1136/ard.2010.148031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerowitz E.A. Transmission of SARS-CoV-2: a review of viral, host, and environmental factors. Ann. Intern. Med. 2020 doi: 10.7326/M20-5008. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongkolpun W. Alterations in skin blood flow at the fingertip are related to mortality in patients with circulatory shock. Crit. Care Med. 2020;48:443–450. doi: 10.1097/CCM.0000000000004177. [DOI] [PubMed] [Google Scholar]
- Orbegozo D. Skin microcirculatory reactivity assessed using a thermal challenge is decreased in patients with circulatory shock and associated with outcome. Ann. Intensive Care. 2018;8:60. doi: 10.1186/s13613-018-0393-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr J.B. Time to reassess tocilizumab’s role in COVID-19 pneumonia. JAMA Intern. Med. 2020 doi: 10.1001/jamainternmed.2020.6557. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- Passali G.C., Bentivoglio A.R. Comment to the article “Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study”. Eur. Arch. Otorhinolaryngol. 2020;277:2391–2392. doi: 10.1007/s00405-020-06024-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrilli C.M. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons S. The vascular endothelium: the cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Crit. Care. 2020;24:353. doi: 10.1186/s13054-020-03062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psotka M.A. Conduct of clinical trials in the era of COVID-19: JACC scientific expert panel. J. Am. Coll. Cardiol. 2020;76:2368–2378. doi: 10.1016/j.jacc.2020.09.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raucci F. Interleukin-17A (IL-17A), a key molecule of innate and adaptive immunity, and its potential involvement in COVID-19-related thrombotic and vascular mechanisms. Autoimmun. Rev. 2020;19:102572. doi: 10.1016/j.autrev.2020.102572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson S. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi M. Skin vasodilator effect of exogenous urotensin-II in hypertensives not exposed to antihypertensive medication. Blood Press. 2008;17:18–25. doi: 10.1080/08037050701757994. [DOI] [PubMed] [Google Scholar]
- Roustit M., Cracowski J.L. Non-invasive assessment of skin microvascular function in humans: an insight into methods. Microcirculation. 2012;19:47–64. doi: 10.1111/j.1549-8719.2011.00129.x. [DOI] [PubMed] [Google Scholar]
- Roustit M., Cracowski J.L. Assessment of endothelial and neurovascular function in human skin microcirculation. Trends Pharmacol. Sci. 2013;34:373–384. doi: 10.1016/j.tips.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Roustit M. Discrepancy between simultaneous digital skin microvascular and brachial artery macrovascular post-occlusive hyperemia in systemic sclerosis. J. Rheumatol. 2008;35:1576–1583. [PMC free article] [PubMed] [Google Scholar]
- Ruan Q. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruaro B. Microvascular damage evaluation in systemic sclerosis: the role of nailfold videocapillaroscopy and laser techniques. Reumatismo. 2017;69:147–155. doi: 10.4081/reumatismo.2017.959. [DOI] [PubMed] [Google Scholar]
- Salgado M.A. Effectiveness of laser Doppler perfusion monitoring in the assessment of microvascular function in patients undergoing on-pump coronary artery bypass grafting. J. Cardiothorac. Vasc. Anesth. 2014;28:1211–1216. doi: 10.1053/j.jvca.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Salton, F., et al., 2020. Prolonged low-dose methylprednisolone in patients with severe COVID-19 pneumonia. Open Forum Infect Dis. 7, ofaa421. [DOI] [PMC free article] [PubMed]
- Sardu C. Hypertension, thrombosis, kidney failure, and diabetes: is COVID-19 an endothelial disease? A comprehensive evaluation of clinical and basic evidence. J. Clin. Med. 2020;9 doi: 10.3390/jcm9051417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorelli M. Assessment of cutaneous microcirculation by laser Doppler flowmetry in type 1 diabetes. Microvasc. Res. 2019;124:91–96. doi: 10.1016/j.mvr.2019.04.002. [DOI] [PubMed] [Google Scholar]
- Spain-Santana T.A. MIP-1 alpha and MIP-1 beta induction by dengue virus. J. Med. Virol. 2001;65:324–330. doi: 10.1002/jmv.2037. [DOI] [PubMed] [Google Scholar]
- Stratton C.W. Pathogenesis-directed therapy of 2019 novel coronavirus disease. J. Med. Virol. 2020 doi: 10.1002/jmv.26610. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- Tibirica E., De Lorenzo A. Importance of the evaluation of systemic microvascular flow and reactivity in critically ill patients with coronavirus disease 2019 - COVID-19. Microvasc. Res. 2020;131:104028. doi: 10.1016/j.mvr.2020.104028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibirica E., De Lorenzo A. 2020. Increased Severity of COVID-19 in People With Obesity: Are We Overlooking Plausible Biological Mechanisms? Obesity (Silver Spring) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J. Current concepts in assessment of microvascular endothelial function using laser Doppler imaging and iontophoresis. Trends Cardiovasc. Med. 2008;18:109–116. doi: 10.1016/j.tcm.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Ugenti V. Microvascular endothelial dysfunction during cardiopulmonary bypass in surgery for correction of cyanotic and acyanotic congenital heart disease. Microvasc. Res. 2018;120:55–58. doi: 10.1016/j.mvr.2018.06.004. [DOI] [PubMed] [Google Scholar]
- Ullyot D.J. Surgical standby for coronary angioplasty. Ann. Thorac. Surg. 1990;50:3–4. doi: 10.1016/0003-4975(90)90069-i. [DOI] [PubMed] [Google Scholar]
- Virdis A., Taddei S. How to evaluate microvascular organ damage in hypertension: assessment of endothelial function. High Blood Press. Cardiovasc. Prev. 2011;18:163–167. doi: 10.2165/11593630-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Wang W. Definition and risks of cytokine release syndrome in 11 critically ill COVID-19 patients with pneumonia: analysis of disease characteristics. J. Infect. Dis. 2020;222:1444–1451. doi: 10.1093/infdis/jiaa387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel L. Laser Doppler flowmetry for assessing localized scleroderma in children. Arthritis Rheum. 2007;56:3489–3495. doi: 10.1002/art.22920. [DOI] [PubMed] [Google Scholar]
- WHO . 2020. Coronavirus Disease 2019 (COVID-19) Dashboard. November 23rd. [Google Scholar]
- Wiersinga W.J. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Yao Z. Immune environment modulation in pneumonia patients caused by coronavirus: SARS-CoV, MERS-CoV and SARS-CoV-2. Aging (Albany NY) 2020;12:7639–7651. doi: 10.18632/aging.103101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zundler S., Neurath M.F. Interleukin-12: functional activities and implications for disease. Cytokine Growth Factor Rev. 2015;26:559–568. doi: 10.1016/j.cytogfr.2015.07.003. [DOI] [PubMed] [Google Scholar]