Figure 2. B7-H4 is tightly regulated by both glycosylation and ubiquitination.
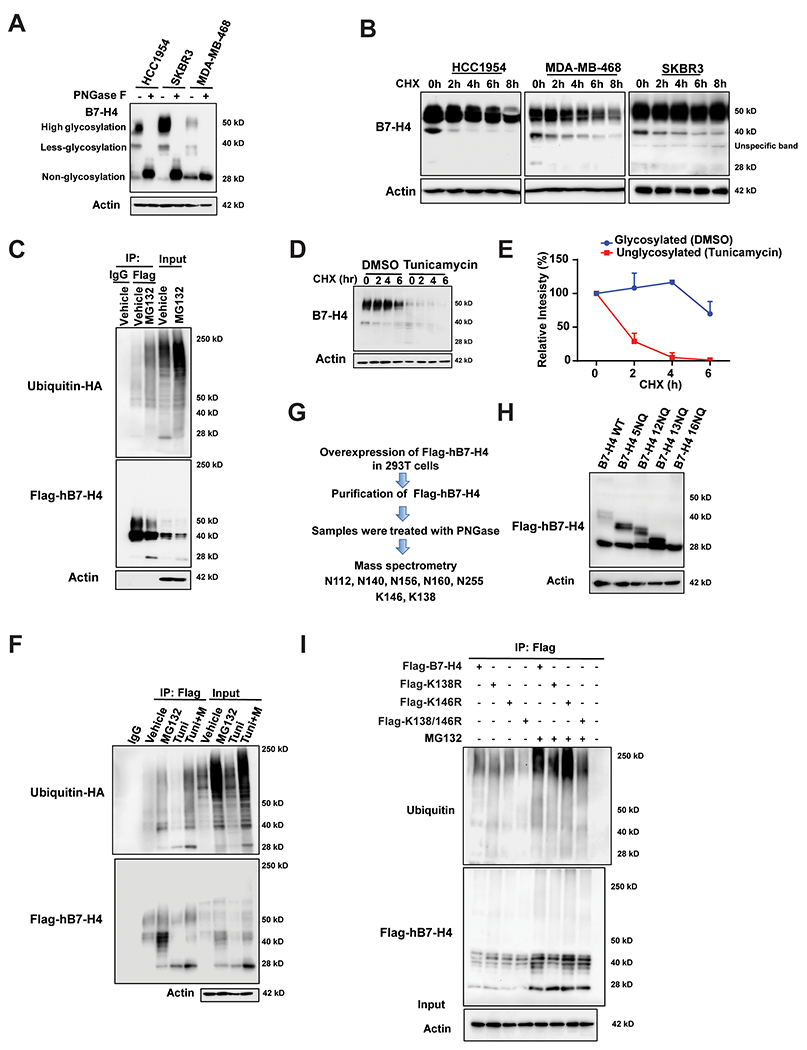
(A) HCC1954, SKBR3 and MDA-MB-468 were treated with PNGase F followed by immunoblot analysis. (B) Pulse-chase analysis for HCC1954, SKBR3 and MDA-MB-468 cells. Cells were treated with 100 μg/ml cycloheximide at the indicated time point. B7-H4 levels were measured by immunoblotting. Actin was used as a loading control. (C) Ubiquitination assay. Flag-hB7-H4 were transfected into 293T cells in the presence or absence of proteasome inhibitor MG132. Then Flag-B7-H4 was immunoprecipitated by anti-Flag M2-beads followed by immunoblot using antibody against ubiquitin. (D) Deglycosylation of B7-H4 enhances its turnover. MDA-MB-468 were treated with 10 μg/ml N-glycosylation inhibitor tunicamycin for 24 h followed by pulse-chase with 100 μg/ml cycloheximide. B7-H4 protein levels at the indicated time points were monitored by immunoblot analysis. (E) The intensity of the 50 kDa form of B7-H4 in DMSO-group vs the 25 kDa form of B7-H4 in the tunicamycin group after the treatment with cycloheximide in MDA-MB-468 cells was quantified using ImageLab. (F) Glycosylation of hB7-H4 antagonizes its ubiquitination. 293T cells were transfected with Flag-hB7-H4 in the presence or absence of MG132 and/or tunicamycin. Flag-hB7-H4 was then immunoprecipitated followed by immunoblotting using anti-ubiquitin antibody. (G) The identification of glycosylation sites on hB7-H4. Flag-hB7-H4 was transfected into 293T cells followed by affinity capture purification in the absence or presence of PNGase F. The indicated glycosylation and ubiquitination sites have been identified by mass spectrometry analysis. (H) A series of glycosylation sites mutants were constructed and validated by sequencing followed by the transfection into 293T cells and immunoblotting. (I) Flag-tagged hB7-H4 wildtype and the mutants (K138R, K146R and K138/K146R (2KR)) were transfected into 293T cells. B7-H4 ubiquitination was conducted in the presence or absence of MG132.
