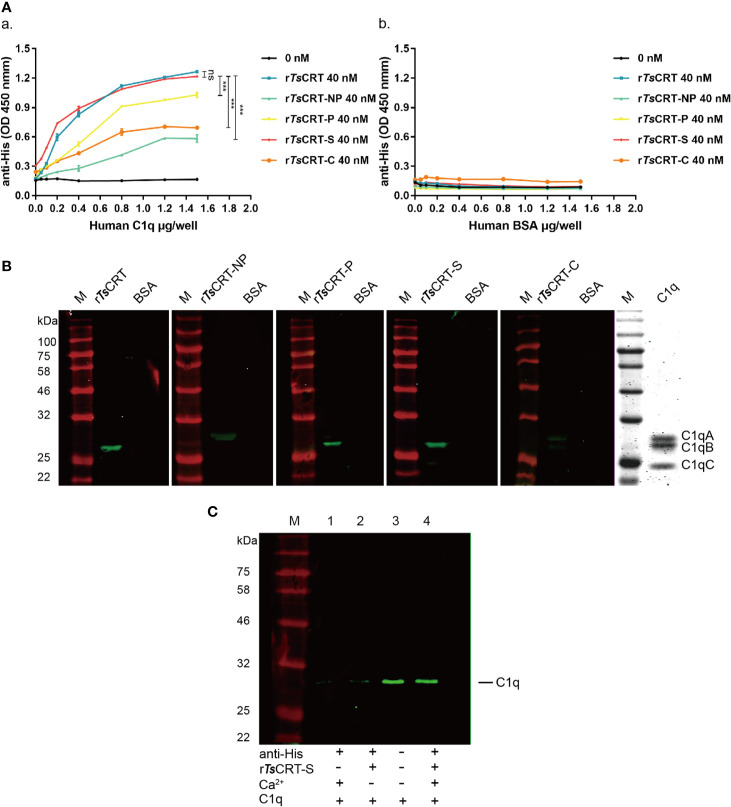
Figure 3.
Human C1q binding capacity of different TsCRT fragments. (A) ELISA measurement of the binding capacities of different rTsCRT fragments to plates coated with human C1q, detected using anti-His antibody (a). rTsCRT and its fragments did not bind to the BSA control (b). Experiments were repeated three times. Data are shown as the mean ± SEM (***p < 0.001). (B) Far western blot showing the binding of C1q with rTsCRT and its fragments. 5 µg of C1q and BSA were transferred to nitrocellulose membranes, then incubated with 5 µg/ml rTsCRT, rTsCRT-NP, rTsCRT-P, rTsCRT-S, and rTsCRT-C and probed with anti-His antibody (1:5,000). C1q was separated by gel electrophoresis to assess the relative molecular weights of the A, B, and C chains. (C) Ca2+-dependent rTsCRT-S binding to C1q. C1q was pulled down by immunoprecipitation using rTsCRT-S bound on anti-His IgG/ProteinG beads in the buffer with and without Ca2+. Eluted complexes were separated by SDS-PAGE and transferred to a nitrocellulose membrane and then detected using a rabbit anti-C1qA antibody. M, molecular weight marker.