Abstract
Background:
Three-dimensional (3D) cell cultures with architectural and biomechanical properties similar to those of natural tissue have been the focus for generating liver tissue. Microarchitectural organization is believed to be crucial to hepatic function, and 3D cell culture technologies have enabled the construction of tissue-like microenvironments, thereby leading to remarkable progress in vitro models of human tissue and organs. Recently, to recapitulate the 3D architecture of tissues, spheroids and organoids have become widely accepted as new practical tools for 3D organ modeling. Moreover, the combination of bioengineering approach offers the promise to more accurately model the tissue microenvironment of human organs. Indeed, the employment of sophisticated bioengineered liver models show long-term viability and functional enhancements in biochemical parameters and disease-orient outcome.
Results:
Various 3D in vitro liver models have been proposed as a new generation of liver medicine. Likewise, new biomedical engineering approaches and platforms are available to more accurately replicate the in vivo 3D microarchitectures and functions of living organs. This review aims to highlight the recent 3D in vitro liver model systems, including micropatterning, spheroids, and organoids that are either scaffold-based or scaffold-free systems. Finally, we discuss a number of challenges that will need to be addressed moving forward in the field of liver tissue engineering for biomedical applications.
Conclusion:
The ongoing development of biomedical engineering holds great promise for generating a 3D biomimetic liver model that recapitulates the physiological and pathological properties of the liver and has biomedical applications.
Keywords: Liver, 3D in vitro liver model, Spheroids, Organoids
Introduction
The study of human liver physiology and disease pathogenesis is limited due to the difficulty of maintaining long-term cultures in vitro and the lack of culture platforms specific to liver microenvironments. Most of the traditional in vitro liver models are created on tissue culture plates, on which cells form a monolayer. Under these conditions, two-dimensional (2D) monolayered cells undergo changes in morphology, hepatic functions, hepatocellular polarity, and genotypic expression due to disturbances to cell-extracellular matrix (ECM) interactions and mechanical-biochemical cues [1–3]. In addition, primary hepatocyte cultures are limited by the inability to maintain cell functionalities, such as albumin production and cytochrome P450 expression, over time [4–6]. Using liver disease models based on 2D cell culture to study the pathogenesis and treatment, the response to an effective therapy can be validated, but the drug screening outcomes are often significantly reduced in clinical practice [7, 8]. To overcome these limitations to in vitro studies, three-dimensional (3D) cell culture that resembles the architectural and biomechanical properties of native tissues has been explored to generate human liver tissue.
To imitate the in vivo properties of the liver, it is essential to understand the anatomical structure and physiology of the organ. The human liver is divided into two main lobes and contains approximately one million hepatic lobules. The portal triads, consisting of the hepatic artery, portal vein, and bile duct, are located at marginal angles to the hepatic lobules. Compared to other organs, the liver has two major sources of blood: one supplies nutrients from the digestive system and the other delivers oxygen from the heart. Even in liver with an attenuated ECM, the ECM plays an important role in the differentiation of liver parenchymal cells and nonparenchymal cells (NPCs). This microarchitectural organization is believed to be crucial to hepatic function, and 3D cell culture technologies have enabled the construction of tissue-like microenvironments that mimic native microarchitectures, thereby leading to remarkable progress in bioengineered human tissue and organs in vitro.
3D cell cultures range from a stack of layered cells in monolayers, spheroids and organoids to far more advanced systems involving biomaterial scaffolds, 3D bioprinting and physiological fluid flow. Multilayered coculture approaches have proven to be effective for capturing the distinct features of the liver in an in vivo microenvironment [9, 10]. However, the random distribution of cellular organization does not precisely mimic the controlled homotypic and heterotypic cell-to-cell interactions of the liver [11]. Micropatterned cocultures with a constant ratio of cells across various patterned configurations control the interactions between homotypic and heterotypic cell populations [12, 13]. Even among those cultures that accommodate well-defined cell-to-cell interactions, there is a challenge associated with cell-substrate adhesiveness. To overcome this major drawback of this system, a range of different substrates have been considered for 3D liver cell patterning.
More recently, to recapitulate the densely packed 3D architecture of tissues, 3D self-organized culture models based on spheroids and organoids are becoming widely accepted as a new practical tool in 3D organ modeling. Spheroids are scaffold-free spherical and heterogeneous self-organizing cell aggregates that can compensate for some of the inadequacies of 2D cultures. Hepatocellular spheroids represent a promising approach for increasing the longevity of parenchymal functions, including albumin secretion, urea synthesis, and phase I and phase II metabolic activities [14, 15]. Organoids can be derived from tissue-resident stem/progenitor cells or pluripotent stem cells that are capable of self-renewal, self-organization and recapitulation of the major features of native tissues.
Recent advances in the field of organoids have led to the generation of human liver organoids from both healthy and diseased tissues, revealing aspects of liver development, biology, and disease in an unprecedented manner [16, 17]. Although 3D cultures, such as spheroids and organoids, can reproduce the histological and biological properties of native tissues, they lack the key features of the natural microenvironments of the stromal, vascular and immune systems.
Differences between spheroids and organoids
The development of a wide range of 3D in vitro cell culture technologies, including spheroids and organoids, has evolved through advances in cell biology, microfabrication technology and tissue engineering. Spheroids and organoids are 3D structures composed of many cells, but they have differences (Fig. 1). A spheroid model was described and later realized through the cultivation of a cancer cell line under non-adherent conditions in the early 1970s [18]. More specifically, tumor spheres are cancer stem cell expansion models derived from tumor spheres in tissue, and organotypic multicellular spheroids are typically obtained by mechanical dissociation and cleavage of tumor tissue [19]. These spheroid models can be generated to develop gradients of oxygen, nutrients, signaling molecules and metabolites by compensating for the deficiencies in 2D culture and through the use of heterogeneous cell populations. In addition, these systems enable geometrical and physiological cell–cell and cell-ECM interactions.
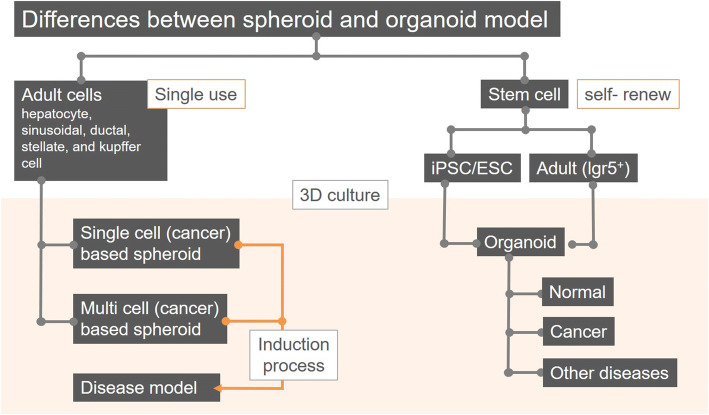
Fig. 1.
Differences between spheroid and organoid as 3D in vitro liver models. As cell sources for 3D spheroid models by cell aggregation, adult cells are mainly used and divided into models such as single cell-based spheroids and multicellular tumor spheroids (MCTs) such as multicellular based spheroids. Single- and multi cell-based spheroid models can be induced into disease models through the administration of chemical substrates and aggregation with stromal cells, respectively. And, cellular sources of the 3D liver model using self-renewing characteristics include the iPSCs/ESCs with pluripotency and Lgr5 positive adult stem cells. Organoids by the 3D culture method of these cells can be generated into normal, cancer and other disease models
Organoids are the collection of organ-specific cells that develop from organ stem cells or progenitors and self-organize through cell sorting and spatially restricted lineage commitment in an manner similar to that in vivo [20]. In recent years, organoids have attracted great attention as 3D in vitro models with superior function. Compared to spheroids, they are more dependent on a matrix. Organoids are classified into tissue and stem cell types based on the way the organ bud is formed [21]. Tissue organoids are grown in mesenchyme-free culture and are mostly comprise epithelial cells due to their inherent ability to self-organize into tissue-like structures. Stem cell organoids are generated from various types of stem cells, including embryonic stem cells or induced pluripotent stem cells or primary stem cells, such as neonatal tissue stem cells and tissue-resident adult stem cells. In addition, various organoids have been developed through numerous different approaches: (1) Organoids are formed from direct transfer of feeder cells that had differentiated in monolayer cultures or on ECM-coated surfaces. (2) Organoids are formed through the use of a mechanically supported culture that promotes the differentiation of primary tissues. (3) Organoids can be generated from embryoid bodies on low-attachment plates or hanging drop plates in methods similar to those used for creating spheroid cultures. (4) Organoids generated from embryonic stem cell are formed by serum-free floating culture methods using embryoid body-like aggregates that reaggregate rapidly on low-attachment plates [22]. Several organoids have been established as functional organoids similar to various tissues, including the pancreas [23], liver [24, 25], stomach [26, 27], intestine [28], lung [29], kidney [30, 31], thyroid [32], thymus [33], cerebral cortex [34] and retina [35].
Although many current organoid cultures do not have the inability to model immune responses due to limitations caused by the lack of a native microenvironment or the inability to promote interaction with immune cells, human cell-derived organoids have the capacity to provide in vivo-like physiological models with which to study human development and human diseases. More advanced organoid cultures enable the development of screening platforms for drug discovery that are more cost-effective than animal models and can provide accurate models of human disease that cannot be replicated in animals. In addition to organoids, patient cancer tissue-derived tumoroids, including cancer cells and stromal cells in the tumor microenvironment (TME), are primed to provide an advanced and more realistic 3D culture platform for personalized drug screening and discovery [36–38]. Figures 2 and 3 show the distinguishing features of the 3D in vitro liver models by two types of model.
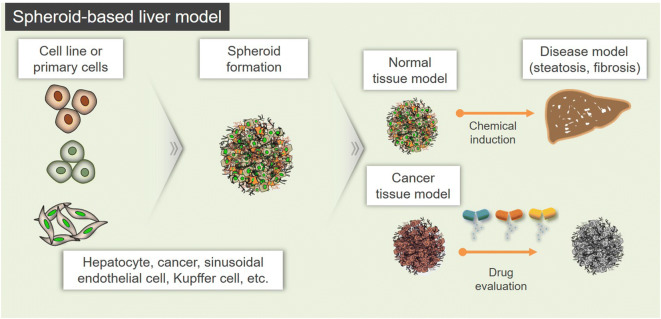
Fig. 2.
Perspectives for application of the spheroid-based liver models. Spheroid-based liver model is formed by aggregation of cell line or primary cells and may be formed to a normal or cancer model. These models can be induced into disease model such as steatosis and fibrosis by chemical induction. Also, cancer model is applicable to study the drug evaluation
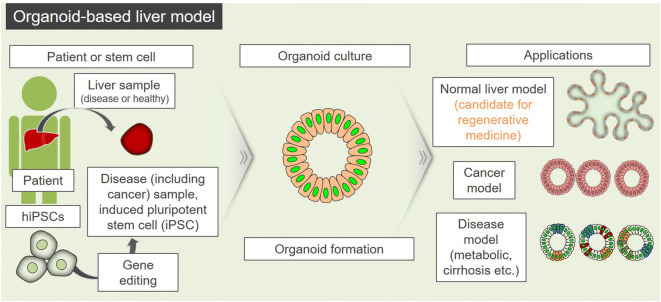
Fig. 3.
Perspectives for application of the organoid-based liver models. Stem cells or patient’s sample-derived cells can be generated into various organoid-based liver models such as cancer, disease, and normal liver. Normal models can be used as candidates for regenerative medicine, and cancer and disease models can be applied for drug screening and evaluation
Spheroid-based 3D liver models
3D growth of cell lines or primary cell cultures has been considered as a more urgent and representative model for the in vitro drug screening [39] (Fig. 2). Cell heterogeneity of 3D multicellular tumor spheroids (MCTs) is similar to that found in avascular micro-regions of tumors and is well established that solid tumor environment induces the drug resistance to many chemotherapeutic agents [40]. This drug resistance by cell heterogeneity occurs as soon as cancer cells have established contacts with surrounding cells or ECM [41]. Then, cancer cells can acquire resistance by 3D interaction such as cell–cell or cell-ECM [41–43]. Thus, 3D MCTs model has been generally considered to predict the in vivo response to drug treatments [44].
Environment for 3D in vitro model with mimicking the in vivo tissue architecture
Primary liver tumor tissues are heterogeneous, consisting of epithelial cells, cancer-associated fibroblasts (CAFs) and other nonepithelial cells. The MCTs designed to study the liver have been further developed to simulate in vivo tumor conditions with a focus on the interaction between cancer cells and various surrounding cells (cancer-associated fibroblast (CAFs), stellate cell, and vascular endothelial cell), mimicking in vivo TME during tumor progression [45–48]. Lau and colleagues formed MCTs using primary hepatocellular carcinoma (HCC) tissue-derived patient cells to examine the potential role of CAFs in the regulation of liver tumor-initiating cells (T-ICs). The results showed that alpha smooth muscle actin (αSMA)-positive CAFs in the presence of hepatocyte growth factor (HGF) regulated T-ICs via the activation of the c-Met/FRA1/HEY1 cascade. Thus, these authors suggested that targeting this signal transduction pathway may be a promising therapeutic approach for the treatment of HCC [46].
In a review, Wang et al. described various stem cell sources and culture technologies (including a 3D organoid culture) through which to introduce stem cell-based therapeutic research for liver diseases [49]. In this review, the authors explained that 3D cultures can produce progenitors with high proliferative capacity. Researchers have also used 3D culture methods to identify hepatic differentiation and maturation of pluripotent stem cells, such as embryonic stem cells (ESCs), induced pluripotent cells (iPSCs) and mesenchymal stem cells [49–51]. Recently, Jeong and colleagues compared human ESCs with human iPSCs as sources of hepatocyte-like cells [52]. They reported that two types of cells expression hepatocyte markers very similarly during differentiation, and the two types of differentiated cells also have similar functional properties. The establishment of a 3D liver spheroid model and organoids based on various types of cells with the capacity to differentiate into hepatocyte-like cells could provide advanced platforms for studying liver diseases.
To achieve nearly physiological models with complex organization that resembles that found in native tissues, 3D cultures such as those based on spheroids have been generated through various approaches. 3D spheroids are multicellular aggregates that exhibit complex cell-to-cell contact and polarity and can be generated from primary cells, stem cells or established cell lines [53–56]. The earliest reports of hepatic spheroids seeded on non-adherent plastic surfaces demonstrated that such 3D aggregates were viable for several weeks and had relatively high function [57, 58]. Different approaches for facilitating spheroid cultures have been previously reported to date, such as scaffold-free and or scaffold-based 3D spheroid cultures and perfused 3D cultures.
Technologies for formation of 3D in vitro models
Ideally, a 3D model should simulate a tissue-specific physiological or pathophysiological microenvironment that includes cell–cell and cell-ECM interactions, tissue-specific stiffness, nutrients and oxygen accessibility, and effective metabolite gradients and that enables cell proliferation, aggregation and differentiation [59]. Scaffold-free, scaffold-based and specialized culture platforms, such as microfluidic devices for 3D culture models, do not meet all the criteria for recreating a functional microenvironment, including those features described above, but they can provide systems with which to form 3D in vitro models for specific applications. In addition, the most recent 3D culture systems are based on advanced 3D culture technologies that make them compatible with automated high-throughput screening (HTS), enabling the discovery of new drug candidates and screening of the other reactions of known drugs in more physiologically accurate cell culture simulations.
Advances in microfabrication technology have enabled desired micropatterns to be printed on the surface of plates. Coated micropatterned plates for non-adherent cells can be designed to promote cell–cell interaction in the scaffold-free 3D microsphere formed within a confined micro-space. Micropatterned plates can also be fabricated to provide a scaffold-based 3D environment that facilitates cell adhesion for the formation of adjacent networks on the surface.
In the hanging drop method, cells are cultured in the free liquid–air interface to generate spheroids after the culture plate lids are inverted [60]. Human 3D spheroidal hepatocellular cultures via the hanging drop technique represent a much more in vivo-like morphology and behavior than shown by monolayer cultures [61]. The hanging drop method has the following advantages: (1) cost effectiveness, (2) controlled spheroid size, and (3) amenability to coculturing with various cell types. However, it is difficult to maintain long-term spheroids due to the limited volume of droplets generated. To produce long-term 3D human liver spheroids, primary hepatocytes were plated on low-binding plates and cultured for 3 days [62]. The spheroids were collected and skewered onto a needle array using scaffold-free 3D bioprinting technology. This unique bioprinted hepatic tissue maintained a wide range of long-lasting liver functions. Furthermore, bioreactors enable the optimization of various parameters, such as dissolved oxygen and pH, and promote high-yield formation of spheroids [63, 64]. A perfused bioreactor system for primary cultures of hepatocytes as spheroids enables the robust formation of hepatic-like microtissue and maintains liver-specific activity and architecture, making it suitable for use in long-term drug testing.
Scaffolds are used to create a physical support system for controlling the cellular microenvironment, and they have the potential to increase the longevity and reproducibility of cell functions in vitro [65, 66]. Various scaffolds have been used to generate hepatic spheroids, including naturally derived scaffolds and synthetically derived polymer scaffolds. Natural scaffolds can be constructed from biological materials such as fibronectin, collagen, laminin, and gelatin, while synthetic components can mimic biological properties of the ECM with high biocompatibility and include poly (lactic acid) (PLA), poly (ethylene glycol) (PEG), poly (vinyl alcohol) (PVA), poly (glycolic acid) (PGA), and polystyrene [67–72]. Chitosan–collagen-coated textile scaffolds enhanced the hepatic functions of primary rat hepatocytes and HepG2 spheroids [73]. Due to the porous nature of the chitosan–collagen matrix, the hepatocyte spheroids received an adequate supply of nutrients and could efficiently exchange substances, such as oxygen and waste, thus enabling the cells to maintain cellular functionality. In a study using synthetically derived polymer scaffolds, biocompatible PEG hydrogel scaffolds that encapsulate primary human hepatocytes within a supportive microenvironment exhibited humanized liver functions that persisted for several weeks and were used to predict the disproportionate metabolism and toxicity of human metabolites [74]. More recently, blended protein-polymer scaffolds have been used to produce consistent, clinically translatable scaffolds as 3D liver cell platforms [75]. THLE-3 cells were cultured in vitro on protein-polymer scaffolds containing human liver ECM, which exerted a significant positive influence on the gene expression profile, albumin production, and liver cell attachment and survival.
Perfusion systems are based on various strategies that allow for automated control over the culture medium pH, fluid pressure, nutrient supply, temperature and process of waste product removal. A perfused liver platform was pioneered by Griffith et al and, called the LiverChip [76, 77]. Hepatocyte aggregates adhere to the collagen-coated walls of microchannels in this device and are then perfused with cell culture medium by integrated pneumatic diaphragm micropumps. Hepatocellular aggregates under these perfusion conditions can maintain hepatocyte-specific functions. Poly (dimethyl siloxane) (PDMS)–based microfluidic devices have also been used to build a 3D liver spheroid model [78, 79]. For instance, concave microwell-based PDMS-membrane-PDMS sandwich multilayer chips can be assembled and disassembled in a simple manner [80]. This biomimetic and reversibly assembled liver-on-a-chip platform (3D-LOC) enables convenient and safe perfusion of cultures comprising hepatic spheroids with a uniform-sized, smooth surface, high cell viability, and low cell loss. The most recent microfluidic strategies have been used to explore the possibility of creating zone-like responses in a microfluid format [81]. The metabolic patterning on a chip (MPOC) consists of a microfluidic gradient generator connected to a microfluidic tissue culture chamber. The metabolic patterning of the liver tissue in the MPOC device can recapitulate some aspects of the liver zonation and zonal toxic responses.
Liver cancer spheroids
The formation of MCTs as representative 3D in vitro tumor models has led to many advances in the use of cancer cell lines or patient-derived cancer cells that target various organs [45–48, 82, 83]. Jung and colleagues fabricated a large and homogenous aggressive liver cancer spheroid by optimizing the MCT formation protocol through a coculture of cells from an HCC cell line (Huh-7) and vascular endothelial cells (HUVECs). In this study, the HUVECs promoted cell proliferation and the expression of HCC-related genes and cancer stem cell markers in the Huh-7-cell based MCTs. In addition, the results of the evaluation showed that the large Huh-7 cell MCTs with HUVECs showed resistance against anti-cancer drugs that was not exhibited by the monolayer cells to which they were compared [45]. To propose a more advanced 3D liver MCT model, Song and colleagues generated a coculture model consisting of hepatitis B virus (HBV)-infected patient-derived HCC cells and human hepatic stellate cells (HSCs), fibroblasts, and HUVECs to screen for potential personalized cancer therapies. They explained that the chemosensitivity assays based on their in vitro patient-derived MCTs may be suitable for advancing the personalized therapy field [48]. To fabricate other types of MCT models, Le and colleagues developed a scaffold-based 3D HCC model that consisted of fibroblasts, HCC cells, and a polycaprolactone (PCL) nanofibrous membrane using three distinct culture methods (monolayer, layered, and mixed) to mimic the in vivo TME. Among the three models, the mixed model resulted in phenotypic changes to the cancer cells and promoted the expression of fibronectin and vimentin. Despite treatment with 100 μM of methotrexate (MTX) drug, the mixed model also showed higher resistance than the other models. The authors suggested that three models may be suitable for use in efficient anti-cancer therapy tests for cancers at various stages [47].
Liver disease spheroids
In addition to tumor models, studies for liver steatosis and fibrosis models as 3D in vitro spheroid disease models have been actively studied. Liver fibrosis refers to the accumulation of matrix proteins related to the wound-healing process. Liver injury induces activation of hepatic stellate cells (HSCs) that secrete ECM proteins in a quiescent state that maintains homeostasis [84, 85]. Based on the liver fibrosis process caused by the activity of HSCs, Lee et al. isolated primary hepatocytes and HSCs from adult rats to form spheroids using concave microwells based fluidic chips for 3D perfusion cultures. 3D in vitro alcoholic liver disease (ALD) spheroid model was fabricated by combination culture and ethanol treatment. In this study, HSCs showed higher expression of αSMA and collagen production than the non-injury group in the ethanol-injured group (60 μl/ml). It demonstrated that HSCs play a role in the recovery process of hepatocytes caused by injury [86]. And, a 3D in vitro liver steatosis spheroid model has been developed for the study of non-alcoholic fatty liver disease (NAFLD) such as steatosis as a disease that can further progress to liver fibrosis. Kozyra et al. formed spheroids on ultra-low attachment (ULA) plates using primary human hepatocytes and induced steatosis through exposure and treatment of lipogenic substrates such as free fatty acids and monosaccharides (glucose and fructose). In this study, 3D spheroids accumulated lipid droplets by exposure to excessive free fatty acids, carbohydrates and insulin levels, and this system showed many in vivo phenomena such as the development of insulin resistance, reversibility of steatosis, and successful treatment [8]. Then, Pingitore et al. formed spheroids on the ULA plate through the physiological ratio “(24: 1)” of hepG2 HCC cell line and LX-2 hepatic stellate cells, and confirmed the accumulation of fat and collagen after exposure to free fatty acids. The authors described the 3D in vitro NAFLD spheroid model was established by confirmation of reducing the accumulation of fat and collagen by treatment of liraglutide or elafibranor as clinical trials for the non-alcoholic steatohepatitis (NASH) [87]. As mentioned, studies on the 3D in vitro spheroid model for the study of fibrosis, steatosis and NAFLD as liver diseases except tumors are evolving.
Organoid-based 3D liver models
Over the last decade, organoid cultures have emerged as promising model systems that can bridge the gap between in vitro and in vivo research. Organoids are 3D cellular clusters derived from primary tissues, ESCs or iPSCs that recapitulate features of the tissue from which the cells originate [20, 88]. Compared with spheroids, organoids generate more tissue/organ level phenotypes with higher order tissue complexity [89]. An early attempt to produce liver bud organoids was based on human iPSCs that share numerous features with fetal liver cells [25]. On the basis of adult somatic tissue-resident stem cell systems, organoid cultures have been shown to sustain long-term expansion, and the expanded cells could be differentiated into functional hepatocytes in vitro [24]. However, such modeling of the human liver in 3D organoids still needs to be tuned and optimized to support the development and maturation of organoid cultures. In this regard, the combination of organoids with a bioengineering-inspired culture system has been used to improve the development of biofabricated tissues for use in personalized medicine and drug screening. The cell sources for liver organoid formation, the development of various models, and their applications are shown in Fig. 3.
Beginning of liver normal organoid models
Fabricated 3D tissues with scaffolds are often the product of 3D cellular aggregates. However, these technologies have been associated some disadvantages, including exogenous origin, potential RNA interference, and batch-to-batch variability [90–92]. A recent study was developed on both in vivo and ex vivo methods for growing in vitro-generated liver buds without scaffolding [93]. In this study, the scaffold-free liver bud-like cell aggregates grown on 3D bioprinter material fused with each other, exhibiting self-tissue organization ex vivo, and could be engrafted into rat liver.
Organoids can be reconstructed by providing appropriate scaffolds and biochemical cues [94, 95]. To date, most organoids are grown on a scaffold-based system based on Matrigel, collagen, synthetic or semisynthetic matrices, etc. [96–98]. A system to obtain liver organoids from epithelial cell adhesion molecule (EpCAM)+ ductal cells was developed by the Clevers group: the EpCAM+ cells were cultured in Matrigel with complete medium containing epidermal growth factor (EGF), HGF, fibroblast growth factor (FGF) and r-spondin-1 (RSPO1) could possibly differentiate toward the hepatic lineage [99]. Alternative approaches have been generated using synthetic matrices that promote organoid generation with less variability and higher reproducibility. Synthetic hydrogel platforms composed of PEG have been recently developed and have been shown to maintain human embryonic stem cell pluripotency and to differentiate to the same extent as cells cultured on Matrigel [97, 100]. Klotz et al. showed that a hybrid hydrogel composed of a gelatin and PEG (gelPEG) platform on which liver‐like tissue analogues were grown had improved liver organoid differentiation, as shown by elevated albumin expression and ALAT and ASAT activity levels [101]. Another interesting application of scaffold-based organoids involves the development of fully defined liver organoid platforms using an inverted colloid crystal (ICC) [102]. 3D and hexagonally arrayed ICC scaffolds have several advantages, including variable mechanical stiffness, functionalization through the use of different ECM proteins, and a homogeneous architecture. The ICC-engineered liver organoids were relatively more similar to those comprising adult tissues with respect to morphology, transcriptomic and protein expression profiles, drug metabolism and viral infection and could integrate into the lobe of murine liver where it was able to vascularize and function following transplantation.
It has been noted that combining organoid cultures with microphysiological systems holds great promise for better mimicking in vivo-like conditions [103, 104]. In an early example, hepatocyte aggregates from primary and iPSC-derived cells were encapsulated in hydrogel droplets and entrapped in a microfluidic device during the hepatoblast expansion phase [105]. On-chip perfusion enabled inducible CYP activity and demonstrated a lifetime of at least 28 days. In a parallel study, Wang et al. showed that liver organoids from human iPSC-based embryoid bodies recapitulated the key features of human liver formation with cellular heterogeneity in a 3D chip system subject to perfusion [106].
Development of various liver disease organoid models
Liver cancer organoids
HCC tumors are also associated with cellular and molecular heterogeneity, chromosomal aberrations, and both somatic and germ line mutations. Wang and colleagues used a multicellular-based organotypic culture system method to investigate the biological features of HCC. HCC cells, fibroblasts, endothelial cells and ECMs were used to form organoid-like spheroids with enhanced characteristics of human HCC in vivo, and nonparenchymal cells were used to retain tissue-like structures. In addition, HCC cells cultured with spheroids in combination with nonparenchymal cells have been shown to express neo-angiogenesis-related markers (vascular endothelial growth factor (VEGF), VEGF receptor 2 (VEGFR2) and hypoxia inducible factor 1 alpha (HIF1α)), tumor-associated inflammatory factors (C-X-C chemokine receptor 4 (CXCR4), C-X-C motif chemokine ligand 12 (CXCL12) and tumor necrosis factor alpha (TNF-α)) and epithelial mesenchymal transition proteins (transforming growth factor beta (TGFβ), vimentin and matrix metallopeptidase 9 (MMP9)) at higher levels than organoids containing only HCC cells. The novelty of this method is related to its suitability for high-throughput approaches useful for identifying HCC malignant tumors and for accurate antitumor therapy screening after surgery [107]. Broutier and colleagues have established liver cancer organoids based on the three most common primary cancer subtypes (HCC, Cholangiocarcinoma (CC) and Combined hepatocellular carcinoma (CHC)) and demonstrated that liver cancer organoids preserve the morphological structures, gene expression levels, and genetic characteristics of the primary cancers. Drug testing using these organoids were used to evaluate the possibility that ERK inhibitor SCH772984 is a novel agent for liver cancer therapy, thus demonstrating that liver cancer organoids can be used for the discovery of new biomarkers and drug screening tests [17]. Recently, Nuciforo and colleagues established organoids from HCC patient-derived needle biopsy samples and compared them with corresponding tumor biopsy samples. These organoids, compared with the original tumor biopsy tissue, showed similarities in terms of growth pattern, differentiation grade, expression profiles of HCC-specific markers, and ability to form tumors in a xenograft model [108]. In these studies, HCC organoids retained genetic alterations as well as chromosomal aberrations [17, 108]. However, some of the organoids appeared to have acquired additional alterations during cultivation [108]. In addition, CC organoids were found to recapitulate most CC biopsy phenotypes, such as solid growth, the presence of atypical cells and intracytoplasmic lumen structures, and mucin production [17, 108]. These results suggest that the success rate of establishing liver cancer organoids from in vivo tumor tissues depends on the rate of tumor cell growth and the differentiation stage of the liver tumor, but it is not affected by the patient’s clinical pathology [17, 108].
Metastatic liver cancer is often formed from the metastasis of malignant gastrointestinal cancers. It leads to poor prognosis and is impossible to treat surgically after it has manifested, but the mechanism of liver metastasis remains unclear. Therefore, the development of a model to study the metastatic mechanisms of liver cancer can provide the opportunity for selecting the optimal chemotherapy to treat metastatic liver cancer that is insensitive to standard chemotherapy. Furthermore, the model using the patient tissues may provide opportunities to customize treatments for individual therapy. Recently, Buzzelli and colleagues generated metastatic colon cancer organoids from liver tissue with colon cancer metastatic tumors [109]. The organoids expressed proteins that are characteristic to primary colon cancer and proteins such as EpCAM and mucin 2 (MUC2) that are expressed in metastatic colon cancer in liver tissues. Additionally, they showed similar chemotherapeutic sensitivity as that of primary colon cancer. On the basis of gene editing technology, experiments in which colon cancer organoids are implanted into nude mice, researchers confirmed that the Trp53 gene could promote liver metastasis [110]. A metastatic liver cancer organoid model may provide opportunities to identify metastatic mechanisms and to develop anticancer drugs.
Liver fibrosis organoids
Liver fibrosis due to progressive accumulation of fibrillar ECM in the liver may result in chronic liver disease (CLD), which is a consequence of repetitive liver tissue damage caused by toxicity from drug use, hepatitis B and C virus infection or metabolic or autoimmune diseases [111]. Currently, liver fibrosis models are being studied through cocultures of hepatic stellate cells (HSCs) and hepatocytes in 2D culture conditions or in 3D culture and/or animal studies, but the low effectiveness of antifibrotic drugs in culture compared with their effectiveness in clinical practice is evident. Therefore, the establishment of an advanced model that can better reflect the pathological process of liver fibrosis in humans is necessary to study the pathogenesis, potential antifibrotic treatments, and drug resistance mechanisms and to verify the effect of antifibrotic drugs for liver fibrosis treatment. For a drug-induced model, Leite and colleagues [112] formed liver organoids by coculturing both HepaRG hepatocytes and HSCs and induced liver fibrosis (LF) using acetaminophen (APAP). These organoids with manifest LF maintained hepatic activity and function and showed cell–cell and cell–matrix interactions [113] that appears during the formation of LF in the body. The researchers found that APAP-induced fibrosis induces hepatocyte damage but not direct activity of HSCs. Then, the researchers established a model of hepatic fibrosis induced by MTX and allyl alcohol [113]. In the in vivo liver tissues, from both mice and humans, the fibrosis process induced Lgr5+ cell proliferation. LF organoid models established by coculturing both Lgr5+ cells and other liver component cells isolated from LF patients would be helpful for the development of individualized antifibrotic drugs for treatment of LF, prevention of LF pathogenesis and improvement of liver function.
Metabolic hepatic disease organoids
The liver is an organ responsible for metabolism in the human body and frequently represents various liver diseases caused by abnormal metabolism. Hepatic metabolic diseases are classified into gene-deficiency metabolic liver diseases such as alpha 1 antitrypsin deficiency (AATD) syndrome and Wilson’s disease [16, 24, 114] and nongene-deficiency metabolic liver diseases such as fatty liver diseases. Advanced models for studying these metabolic diseases require tissues with a high degree of structure, gene expression and metabolism similarity with human liver disease tissues.
As a gene-deficiency metabolic liver disease, AATD syndrome caused by the mutation of the SYPANA1 gene leads to abnormal accumulation of antitrypsin in hepatocytes [115] and results in a clinical chronic liver disease syndrome. AATD patient-derived liver organoids expressed albumin and presented with the low-density lipoprotein (LDL)-like features of normal liver tissues. However, they showed abnormal accumulation of antitrypsin and a significant reduction in the hepatocellular excretion of antitrypsin. These characteristics were similar to the liver metabolism in AATD syndrome, and the liver organoids were found to be a good in vitro model for studying AATD syndrome [16]. Wilson’s disease is a genetic disease caused by the loss of COMMD1 [116]. To generate this copper storage disease model, Nantasnti and colleagues established liver organoids from a patient’s diseased liver tissue and found abnormal copper accumulation in the organoids [114]. iPSC-derived liver organoids maintained hepatic tissue regeneration, patient metabolism and intrahepatic bile duct epithelial gene expression [24, 114].
Nonalcoholic fatty liver disease is a major among nongene-deficiency metabolic liver disease that induces liver failure, and the number of cases has risen in the past few decades [117]. A good in vitro model for studying nonalcoholic fatty liver disease is necessary to effectively evaluate lipid absorption disorders in the liver in the future. Nantasnti and colleagues proposed using liver organoids to study nonalcoholic fatty liver and nonalcoholic hepatitis. Kruitwagen and colleagues established liver organoids from specimens of various species, including cats, dogs and humans. Their liver organoids showed differentiation of mature hepatocytes with accumulated glycogen that secreted albumin and expressed cytochrome family molecules [118]. The hepatocytes in the liver organoids ingested a large amount of fatty acids when a sufficient amount of fatty acids was added to the medium. The researchers found differences in the regulation of lipid metabolic pathways between dog liver organoids and human liver organoids. They also been verified that the human fatty liver and dog fatty liver markedly differed in pathology and pathogenesis [118]. However, the mechanism of the fatty acid uptake and the induction of signaling pathways for the regulation of lipid metabolism by hepatocytes has not been studied. Therefore, the liver organoid model may be a valuable research model for studying gene- and non-gene-deficiency metabolic liver diseases.
Chronic viral hepatitis organoids
Chronic viral hepatitis is currently the most common liver disease caused by infection with HBV or hepatitis C virus (HCV) worldwide [119]. The in vitro organoids provide a platform to study the pathogenesis of infectious diseases [120] and have been used to study the intestine infected by rotavirus [121] or norovirus [122–124]. Baktash and colleagues established a liver organoid system capable of polarizing hepatocytes and expressing viral sites and showed that the HCV infection titer in the hepatocytes in this organoid was significantly higher than that of those in the 2D cultures [125]. They reproduced the unusual and complex process of HCV infection in hepatocytes in the organoids and found that EGF receptor (EGFR) is required to achieve particle internalization but is not involved in inducing localization of the HCV. No studies have yet been conducted to study HBV-infected liver cells using liver organoids. Accordingly, liver organoids may constitute a valuable model to use in studying the infection and drug resistance mechanisms of viral hepatitis and in developing drugs to treat viral hepatitis.
Conclusions and future perspectives
Liver plays major biological roles including serum protein secretion, bile production, detoxification, and metabolic homeostasis. Thus, primary hepatocytes of the liver are widely used as in vitro study models and as donor cells for transplantation and bioartificial liver devices [126, 127]. However, adult hepatocytes have limited proliferative capacity and rapidly lose their characteristic phenotype [24, 93], including morphology, when cultured in a monolayer [128, 129]. To circumvent this limitation, a variety of culture technologies for culturing hepatocytes have been developed to achieve a better liver model, including (i) micropatterned cultures with photolithography, (ii) scaffold‐free or scaffold‐based 3D spheroid/organoid cultures, and (iii) biomechanical stimulation produced by culture medium perfusion [12, 60, 65, 76, 130]. For example, vertical flow microfluidic device has been developed to exhibit hepatocyte polarity and enhance hepatocyte functions in prolonged 3D culture by mimicking physiology conditions [131]. Therefore, considerable effort has been dedicated to the creation of a 3D cell-based hepatic model through tissue engineering strategies that preserve hepatocyte‐specific functions for a long period.
There is no doubt that 3D cell culture models bridge the translational gap between in vitro and in vivo models. A wide range of 3D cell culture technologies has been developed to overcome biological and clinical challenges. Currently, sophisticated models of bioengineered liver show high sensitivity such that clinically relevant drug- and disease-oriented outcomes can be determined. In this review, we present an overview of different 3D in vitro liver models that have been recently proposed to study liver-based tissue engineering, including micropatterning, spheroids, and organoids that are either scaffold-based or scaffold-free systems. Finally, we discuss the key parameters that will need to be addressed in the field of liver tissue engineering for enabling future in vitro biomedical applications (Fig. 4).
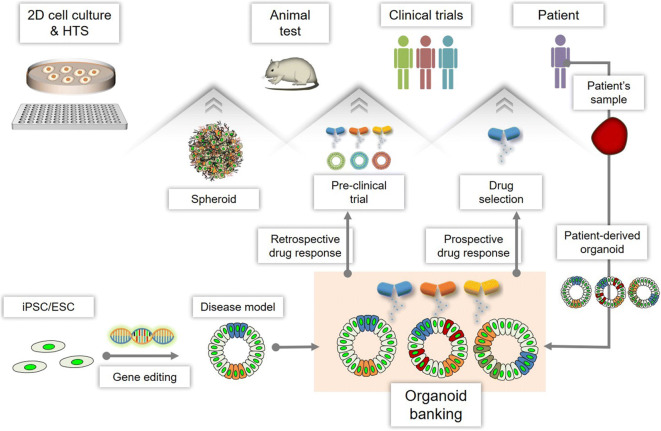
Fig. 4.
Current and future applications of 3D in vitro models. 3D spheroid models by cell aggregation of cell line or primary cells can be used as models for bridge roles in 2D culture methods and animal studies. And, as a more advanced 3D model, organoids can be used for pre-clinical trials for the retrospective drug response. Organoid banking by the generation of patient’s sample- or iPSC/ESC-derived organoids allows the pre-clinical trial as a mid-term study which bridge between animal test and clinical trials through a retrospective drug response. Also, it is capable to get the selection of sensitive drugs by prospective drug response and develop customized treatment
Advances in 3D cell culture technologies have been made possible by simultaneous advances in 3D tissue engineering, such as control of the 3D microenvironment and fabrication of scaffolds as templates for cell aggregate formation [132–134]. Due to improved cell–cell and cell-ECM interactions, the cells cultured in 3D systems have generated nearly physiological models reflecting the architectural and functional properties of primary tissue to a greater extent than 2D cultures. Moreover, novel engineering strategies, such as the addition of a vasculature-like system and creation of multi-organoid cocultures for incorporation within 3D environments generated by cell aggregates, enable the development of more sophisticated 3D cell culture models [135, 136]. For instance, an anteroposterior interactions has been developed to establish an interconnected multi-organ [136]. Herein, the boundary interactions between anterior and posterior spheroids generate hepato-biliary-pancreatic organoids in the absence of extrinsic signaling cues.
3D in vitro liver models are needed to, relatively more culture medium, nutrition and oxygen, compare to that of 2D culture condition. It is one of main reason for limitation of well-define 3D in vitro liver model. Recently, a microfluidic device has been designed to create cell cultures under perfusion with reliable supplies of oxygen and nutrients simultaneous removal of waste. Microfluidic devices can be fabricated to mimic the shear forces found in vivo, such as that on endothelial cells that are exposed to the bloodstream. Additionally, compartment barriers can be physically incorporated into the microfluidic device with nonphysical materials, such as a supporting matrix that mimics the ECM. Microfluidic devices enable the continuous application of drugs, various soluble molecules such as growth factors, or the exchange of fluid between different compartments that may involve different types of cells. These microfluidic devices can be used for long-term tumor cell culture [137–139] and can be designed as automated microfluidic ECM screening platforms with small molecule-screening capability [140]. In addition, microfabrication technology of these microfluidic devices has enabled the development of organ-on-a-chip platforms in 3D models that simulate various organs. It is anticipated that the development of these organ-on-a-chip platforms will support the generation of advanced tools for drug discovery and HTS in the future [141, 142].
Despite advances in 3D cell-based hepatic modeling in vitro, the use of 3D in vitro liver models, including those based on micropatterning, spheroids, and organoids, remains challenging. These models lack the requisite cellular composition and microstructural complexity needed to achieve physiologically relevant levels of organ response [143, 144]. Another major limitation relates to extracellular matrix substitutes in the widely used materials for 3D cell culture, which are of often undefined composition and introduce variables of uncertainly, thus posing limits to the use of 3D in vitro liver models in regenerative medicine and other clinical applications [145, 146]. Nonetheless, the ongoing development of biomedical engineering holds great promise for the generation of 3D biomimetic liver models that recapitulate the physiological and pathological properties of the liver and have biomedical applications.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF-2019R1A2C2005244), the Post-Genome Technology Development Program (10067796) and the Technology Innovation Program (10067407) funded by the Ministry of Trade, Industry and Energy (MOTIE, Korea). SWL contributed to the substantial contribution to the conception and design of the study, or acquisition, interpretation and analysis of data; drafting the article or revising it critically for the important intellectual content; DJJ contributed to the substantial contribution to the conception and design of the study, or acquisition, interpretation and analysis of data; drafting the article or revising it critically for the important intellectual content; and GSJ contributed to the substantial contribution to the conception and design of the study, or acquisition, interpretation and analysis of data;drafting the article or revising it critically for the important intellectual content; final approval of the version to be published.
Compliance with ethical standards
Conflict of interest
The authors have no financial conflicts of interest.
Ethical statement
There are no animal experiments carried out for this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sang Woo Lee and Da Jung Jung have contributed equally to this work.
References
- 1.Duval K, Grover H, Han LH, Mou Y, Pegoraro AF, Fredberg J, et al. Modeling physiological events in 2D vs. 3D cell culture. Physiology (Bethesda) 2017;32:266–277. doi: 10.1152/physiol.00036.2016. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 2.Kapałczyńska M, Kolenda T, Przybyla W, Zajaczkowska M, Teresiak A, Filas V, et al. 2D and 3D cell cultures—a comparison of different types of cancer cell cultures. Arch Med Sci. 2018;14:910–919. doi: 10.5114/aoms.2016.63743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeigerer A, Wuttke A, Marsico G, Seifert S, Kalaidzidis Y, Zerial M. Functional properties of hepatocytes in vitro are correlated with cell polarity maintenance. Exp Cell Res. 2017;350:242–252. doi: 10.1016/j.yexcr.2016.11.027. [DOI] [PubMed] [Google Scholar]
- 4.Lázaro CA, Croager EJ, Mitchell C, Campbell JS, Yu C, Foraker J, et al. Establishment, characterization, and long-term maintenance of cultures of human fetal hepatocytes. Hepatology. 2003;38:1095–1106. doi: 10.1053/jhep.2003.50448. [DOI] [PubMed] [Google Scholar]
- 5.Bell CC, Dankers ACA, Lauschke VM, Sison-Young R, Jenkins R, Rowe C, et al. Comparison of hepatic 2D sandwich cultures and 3D spheroids for long-term toxicity applications: a multicenter study. Toxicol Sci. 2018;162:655–666. doi: 10.1093/toxsci/kfx289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katsura N, Ikai I, Mitaka T, Shiotani T, Yamanokuchi S, Sugimoto S, et al. Long-term culture of primary human hepatocytes with preservation of proliferative capacity and differentiated functions. J Surg Res. 2002;106:115–123. doi: 10.1006/jsre.2002.6446. [DOI] [PubMed] [Google Scholar]
- 7.Cole BK, Feaver RE, Wamhoff BR, Dash A. Non-alcoholic fatty liver disease (NAFLD) models in drug discovery. Expert Opin Drug Discov. 2018;13:193–205. doi: 10.1080/17460441.2018.1410135. [DOI] [PubMed] [Google Scholar]
- 8.Kozyra M, Johansson I, Nordling Å, Ullah S, Lauschke VM, Ingelman-Sundberg M. Human hepatic 3D spheroids as a model for steatosis and insulin resistance. Sci Rep. 2018;8:14297. doi: 10.1038/s41598-018-32722-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas RJ, Bhandari R, Barrett DA, Bennett AJ, Fry JR, Powe D, et al. The effect of three-dimensional co-culture of hepatocytes and hepatic stellate cells on key hepatocyte functions in vitro. Cells Tissues Organs. 2005;181:67–79. doi: 10.1159/000091096. [DOI] [PubMed] [Google Scholar]
- 10.Kostadinova R, Boess F, Applegate D, Suter L, Weiser T, Singer T, et al. A long-term three dimensional liver co-culture system for improved prediction of clinically relevant drug-induced hepatotoxicity. Toxicol Appl Pharmacol. 2013;268:1–16. doi: 10.1016/j.taap.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Bhatia SN, Balis UJ, Yarmush ML, Toner M. Microfabrication of hepatocyte/fibroblast co-cultures: role of homotypic cell interactions. Biotechnol Prog. 1998;14:378–387. doi: 10.1021/bp980036j. [DOI] [PubMed] [Google Scholar]
- 12.Khetani SR, Bhatia SN. Microscale culture of human liver cells for drug development. Nat Biotechnol. 2008;26:120–126. doi: 10.1038/nbt1361. [DOI] [PubMed] [Google Scholar]
- 13.Cho CH, Park J, Tilles AW, Berthiaume F, Toner M, Yarmush ML. Layered patterning of hepatocytes in co-culture systems using microfabricated stencils. Biotechniques. 2010;48:47–52. doi: 10.2144/000113317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arakawa H, Kamioka H, Jomura T, Koyama S, Idota Y, Yano K, et al. Preliminary evaluation of three-dimensional primary human hepatocyte culture system for assay of drug-metabolizing enzyme-inducing potential. Biol Pharmaceut Bull. 2017;40:967–974. doi: 10.1248/bpb.b16-00885. [DOI] [PubMed] [Google Scholar]
- 15.No da Y, Jeong GS, Lee SH. Immune-protected xenogeneic bioartificial livers with liver-specific microarchitecture and hydrogel-encapsulated cells. Biomaterials. 2014;35:8983–8991. doi: 10.1016/j.biomaterials.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 16.Huch M, Gehart H, van Boxtel R, Hamer K, Blokzijl F, Verstegen MM, et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell. 2015;160:299–312. doi: 10.1016/j.cell.2014.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Broutier L, Mastrogiovanni G, Verstegen MM, Francies HE, Gavarró LM, Bradshaw CR, et al. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat Med. 2017;23:1424–1435. doi: 10.1038/nm.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sutherland RM, McCredie JA, Inch WR. Growth of multicell spheroids in tissue culture as a model of nodular carcinomas. J Natl Cancer Inst. 1971;46:113–120. [PubMed] [Google Scholar]
- 19.Weiswald LB, Bellet D, Dangles-Marie V. Spherical cancer models in tumor biology. Neoplasia. 2015;17:1–15. doi: 10.1016/j.neo.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014;345:1247125. doi: 10.1126/science.1247125. [DOI] [PubMed] [Google Scholar]
- 21.Huch M, Koo BK. Modeling mouse and human development using organoid cultures. Development. 2015;142:3113–3125. doi: 10.1242/dev.118570. [DOI] [PubMed] [Google Scholar]
- 22.Turner DA, Baillie-Johnson P, Martinez Arias A. Organoids and the genetically encoded self-assembly of embryonic stem cells. Bioessays. 2016;38:181–191. doi: 10.1002/bies.201500111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greggio C, De Franceschi F, Figueiredo-Larsen M, Gobaa S, Ranga A, Semb H, et al. Artificial three-dimensional niches deconstruct pancreas development in vitro. Development. 2013;140:4452–4462. doi: 10.1242/dev.096628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huch M, Dorrell C, Boj SF, van Es JH, Li VS, van de Wetering M, et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 2013;494:247–250. doi: 10.1038/nature11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499:481–484. doi: 10.1038/nature12271. [DOI] [PubMed] [Google Scholar]
- 26.Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 27.Stange DE, Koo BK, Huch M, Sibbel G, Basak O, Lyubimova A, et al. Differentiated Troy + chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell. 2013;155:357–368. doi: 10.1016/j.cell.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–109. doi: 10.1038/nature09691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JH, Bhang DH, Beede A, Huang TL, Stripp BR, Bloch KD, et al. Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1-thrombospondin-1 axis. Cell. 2014;156:440–455. doi: 10.1016/j.cell.2013.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freedman BS, Brooks CR, Lam AQ, Fu H, Morizane R, Agrawal V, et al. Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat Commun. 2015;6:8715. doi: 10.1038/ncomms9715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature. 2015;526:564–568. doi: 10.1038/nature15695. [DOI] [PubMed] [Google Scholar]
- 32.Antonica F, Kasprzyk DF, Opitz R, Iacovino M, Liao XH, Dumitrescu AM, et al. Generation of functional thyroid from embryonic stem cells. Nature. 2012;491:66–71. doi: 10.1038/nature11525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bredenkamp N, Ulyanchenko S, O’Neill KE, Manley NR, Vaidya HJ, Blackburn CC. An organized and functional thymus generated from FOXN1-reprogrammed fibroblasts. Nat Cell Biol. 2014;16:902–908. doi: 10.1038/ncb3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, et al. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakano T, Ando S, Takata N, Kawada M, Muguruma K, Sekiguchi K, et al. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell. 2012;10:771–785. doi: 10.1016/j.stem.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 36.Pauli C, Hopkins BD, Prandi D, Shaw R, Fedrizzi T, Sboner A, et al. Personalized in vitro and in vivo cancer models to guide precision medicine. Cancer Discov. 2017;7:462–477. doi: 10.1158/2159-8290.CD-16-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stadler M, Walter S, Walzl A, Kramer N, Unger C, Scherzer M, et al. Increased complexity in carcinomas: analyzing and modeling the interaction of human cancer cells with their microenvironment. Semin Cancer Biol. 2015;35:107–124. doi: 10.1016/j.semcancer.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 38.Xu X, Farach-Carson MC, Jia X. Three-dimensional in vitro tumor models for cancer research and drug evaluation. Biotechnol Adv. 2014;32:1256–1268. doi: 10.1016/j.biotechadv.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thoma CR, Zimmermann M, Agarkova I, Kelm JM, Krek W. 3D cell culture systems modeling tumor growth determinants in cancer target discovery. Adv Drug Deliv Rev. 2014;69–70:29–41. doi: 10.1016/j.addr.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 40.Sutherland RM. Cell and environment interactions in tumor microregions: the multicell spheroid model. Science. 1988;240:177–184. doi: 10.1126/science.2451290. [DOI] [PubMed] [Google Scholar]
- 41.Desoize B, Jardillier J. Multicellular resistance: a paradigm for clinical resistance? Crit Rev Oncol Hematol. 2000;36:193–207. doi: 10.1016/s1040-8428(00)00086-x. [DOI] [PubMed] [Google Scholar]
- 42.Mellor HR, Ferguson DJ, Callaghan R. A model of quiescent tumour microregions for evaluating multicellular resistance to chemotherapeutic drugs. Br J Cancer. 2005;93:302–309. doi: 10.1038/sj.bjc.6602710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fayad W, Brnjic S, Berglind D, Blixt S, Shoshan MC, Berndtsson M, et al. Restriction of cisplatin induction of acute apoptosis to a subpopulation of cells in a three-dimensional carcinoma culture model. Int J Cancer. 2009;125:2450–2455. doi: 10.1002/ijc.24627. [DOI] [PubMed] [Google Scholar]
- 44.Kunz-Schughart LA, Freyer JP, Hofstaedter F, Ebner R. The use of 3-D cultures for high-throughput screening: the multicellular spheroid model. J Biomol Screen. 2004;9:273–285. doi: 10.1177/1087057104265040. [DOI] [PubMed] [Google Scholar]
- 45.Jung HR, Kang HM, Ryu JW, Kim DS, Noh KH, Kim ES, et al. Cell spheroids with enhanced aggressiveness to mimic human liver cancer in vitro and in vivo. Sci Rep. 2017;7:10499. doi: 10.1038/s41598-017-10828-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lau EY, Lo J, Cheng BY, Ma MK, Lee JM, Ng JK, et al. Cancer-associated fibroblasts regulate tumor-initiating cell plasticity in hepatocellular carcinoma through c-Met/FRA1/HEY1 signaling. Cell Rep. 2016;15:1175–1189. doi: 10.1016/j.celrep.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 47.Le BD, Kang D, Yun S, Jeong YH, Kwak JY, Yoon S, et al. Three-dimensional hepatocellular carcinoma/fibroblast model on a nanofibrous membrane mimics tumor cell phenotypic changes and anticancer drug resistance. Nanomaterials (Basel) 2018;8:E64. doi: 10.3390/nano8020064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song Y, Kim JS, Kim SH, Park YK, Yu E, Kim KH, et al. Patient-derived multicellular tumor spheroids towards optimized treatment for patients with hepatocellular carcinoma. J Exp Clin Cancer Res CR. 2018;37:109. doi: 10.1186/s13046-018-0752-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang J, Sun M, Liu W, Li Y, Li M. Stem cell-based therapies for liver diseases: an overview and update. Tissue Eng Regen Med. 2019;16:107–118. doi: 10.1007/s13770-019-00178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim JH, Jang YJ, An SY, Son J, Lee J, Lee G, et al. Enhanced metabolizing activity of human ES cell-derived hepatocytes using a 3D culture system with repeated exposures to xenobiotics. Toxicol Sci. 2015;147:190–206. doi: 10.1093/toxsci/kfv121. [DOI] [PubMed] [Google Scholar]
- 51.Kim SE, An SY, Woo DH, Han J, Kim JH, Jang YJ, et al. Engraftment potential of spheroid-forming hepatic endoderm derived from human embryonic stem cells. Stem Cells Dev. 2013;22:1818–1829. doi: 10.1089/scd.2012.0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jeong J, Kim KN, Chung MS, Kim HJ. Functional comparison of human embryonic stem cells and induced pluripotent stem cells as sources of hepatocyte-like cells. Tissue Eng Regen Med. 2016;13:740–749. doi: 10.1007/s13770-016-0094-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Z, Li W, Jing H, Ding M, Fu G, Yuan T, et al. Generation of hepatic spheroids using human hepatocyte-derived liver progenitor-like cells for hepatotoxicity screening. Theranostics. 2019;9:6690–6705. doi: 10.7150/thno.34520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coll M, Perea L, Boon R, Leite SB, Vallverdú J, Mannaerts I, et al. Generation of hepatic stellate cells from human pluripotent stem cells enables in vitro modeling of liver fibrosis. Cell Stem Cell. 2018;23:101.e7–113.e7. doi: 10.1016/j.stem.2018.05.027. [DOI] [PubMed] [Google Scholar]
- 55.Bell CC, Hendriks DF, Moro SM, Ellis E, Walsh J, Renblom A, et al. Characterization of primary human hepatocyte spheroids as a model system for drug-induced liver injury, liver function and disease. Sci Rep. 2016;6:25187. doi: 10.1038/srep25187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vorrink SU, Zhou Y, Ingelman-Sundberg M, Lauschke VM. Prediction of drug-induced hepatotoxicity using long-term stable primary hepatic 3D spheroid cultures in chemically defined conditions. Toxicol Sci. 2018;163:655–665. doi: 10.1093/toxsci/kfy058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Landry J, Bernier D, Ouellet C, Goyette R, Marceau N. Spheroidal aggregate culture of rat liver cells: histotypic reorganization, biomatrix deposition, and maintenance of functional activities. J Cell Biol. 1985;101:914–923. doi: 10.1083/jcb.101.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koide N, Shinji T, Tanabe T, Asano K, Kawaguchi M, Sakaguchi K, et al. Continued high albumin production by multicellular spheroids of adult rat hepatocytes formed in the presence of liver-derived proteoglycans. Biochem Biophys Res Commun. 1989;161:385–391. doi: 10.1016/0006-291x(89)91609-4. [DOI] [PubMed] [Google Scholar]
- 59.Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol. 2006;7:211–224. doi: 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- 60.Foty R. A simple hanging drop cell culture protocol for generation of 3D spheroids. J Vis Exp. 2011 doi: 10.3791/2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Elje E, Hesler M, Rundén-Pran E, Mann P, Mariussen E, Wagner S, et al. The comet assay applied to HepG2 liver spheroids. Mutat Res. 2019;845:403033. doi: 10.1016/j.mrgentox.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 62.Kizawa H, Nagao E, Shimamura M, Zhang G, Torii H. Scaffold-free 3D bio-printed human liver tissue stably maintains metabolic functions useful for drug discovery. Biochem Biophys Rep. 2017;10:186–191. doi: 10.1016/j.bbrep.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shi W, Kwon J, Huang Y, Tan J, Uhl CG, He R, et al. Facile tumor spheroids formation in large quantity with controllable size and high uniformity. Sci Rep. 2018;8:6837. doi: 10.1038/s41598-018-25203-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tostões RM, Leite SB, Serra M, Jensen J, Björquist P, Carrondo MJ, et al. Human liver cell spheroids in extended perfusion bioreactor culture for repeated-dose drug testing. Hepatology. 2012;55:1227–1236. doi: 10.1002/hep.24760. [DOI] [PubMed] [Google Scholar]
- 65.Ruoß M, Häussling V, Schügner F, Olde Damink LHH, Lee SML, Ge L, et al. A standardized collagen-based scaffold improves human hepatocyte shipment and allows metabolic studies over 10 days. Bioengineering (Basel) 2018;5:E86. doi: 10.3390/bioengineering5040086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ovsianikov A, Khademhosseini A, Mironov V. The synergy of scaffold-based and scaffold-free tissue engineering strategies. Trends Biotechnol. 2018;36:348–357. doi: 10.1016/j.tibtech.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 67.Wang Y, Kim MH, Shirahama H, Lee JH, Ng SS, Glenn JS, et al. ECM proteins in a microporous scaffold influence hepatocyte morphology, function, and gene expression. Sci Rep. 2016;6:37427. doi: 10.1038/srep37427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li Y, Kumacheva E. Hydrogel microenvironments for cancer spheroid growth and drug screening. Sci Adv. 2018;4:eaas8998. doi: 10.1126/sciadv.aas8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Afewerki S, Sheikhi A, Kannan S, Ahadian S, Khademhosseini A. Gelatin-polysaccharide composite scaffolds for 3D cell culture and tissue engineering: towards natural therapeutics. Bioeng Transl Med. 2019;4:96–115. doi: 10.1002/btm2.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Polonio-Alcalá E, Rabionet M, Gallardo X, Angelats D, Ciurana J, Ruiz-Martínez S, et al. PLA electrospun scaffolds for three-dimensional triple-negative breast cancer cell culture. Polymers (Basel) 2019;11:E916. doi: 10.3390/polym11050916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee BH, Kim MH, Lee JH, Seliktar D, Cho NJ, Tan LP. Modulation of Huh7.5 spheroid formation and functionality using modified PEG-based hydrogels of different stiffness. PLoS One. 2015;10:e0118123. doi: 10.1371/journal.pone.0118123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Knight E, Przyborski S. Advances in 3D cell culture technologies enabling tissue-like structures to be created in vitro. J Anat. 2015;227:746–756. doi: 10.1111/joa.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Risbud MV, Karamuk E, Schlosser V, Mayer J. Hydrogel-coated textile scaffolds as candidate in liver tissue engineering: II. Evaluation of spheroid formation and viability of hepatocytes. J Biomater Sci Polym Ed. 2003;14:719–731. doi: 10.1163/156856203322274969. [DOI] [PubMed] [Google Scholar]
- 74.Chen AA, Thomas DK, Ong LL, Schwartz RE, Golub TR, Bhatia SN. Humanized mice with ectopic artificial liver tissues. Proc Natl Acad Sci U S A. 2011;108:11842–11847. doi: 10.1073/pnas.1101791108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grant R, Hallett J, Forbes S, Hay D, Callanan A. Blended electrospinning with human liver extracellular matrix for engineering new hepatic microenvironments. Sci Rep. 2019;9:6293. doi: 10.1038/s41598-019-42627-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Powers MJ, Domansky K, Kaazempur-Mofrad MR, Kalezi A, Capitano A, Upadhyaya A, et al. A microfabricated array bioreactor for perfused 3D liver culture. Biotechnol Bioeng. 2002;78:257–269. doi: 10.1002/bit.10143. [DOI] [PubMed] [Google Scholar]
- 77.Domansky K, Inman W, Serdy J, Dash A, Lim MH, Griffith LG. Perfused multiwell plate for 3D liver tissue engineering. Lab Chip. 2010;10:51–58. doi: 10.1039/b913221j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bhise NS, Manoharan V, Massa S, Tamayol A, Ghaderi M, Miscuglio M, et al. A liver-on-a-chip platform with bioprinted hepatic spheroids. Biofabrication. 2016;8:014101. doi: 10.1088/1758-5090/8/1/014101. [DOI] [PubMed] [Google Scholar]
- 79.Yu F, Deng R, Hao Tong W, Huan L, Chan Way N, IslamBadhan A, et al. A perfusion incubator liver chip for 3D cell culture with application on chronic hepatotoxicity testing. Sci Rep. 2017;7:14528. doi: 10.1038/s41598-017-13848-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ma LD, Wang YT, Wang JR, Wu JL, Meng XS, Hu P, et al. Design and fabrication of a liver-on-a-chip platform for convenient, highly efficient, and safe in situ perfusion culture of 3D hepatic spheroids. Lab Chip. 2018;18:2547–2562. doi: 10.1039/c8lc00333e. [DOI] [PubMed] [Google Scholar]
- 81.Kang YBA, Eo J, Mert S, Yarmush ML, Usta OB. Metabolic patterning on a chip: towards in vitro liver zonation of primary rat and human hepatocytes. Sci Rep. 2018;8:8951. doi: 10.1038/s41598-018-27179-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ham SL, Thakuri PS, Plaster M, Li J, Luker KE, Luker GD, et al. Three-dimensional tumor model mimics stromal—breast cancer cells signaling. Oncotarget. 2018;9:249–267. doi: 10.18632/oncotarget.22922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jeppesen M, Hagel G, Glenthoj A, Vainer B, Ibsen P, Harling H, et al. Short-term spheroid culture of primary colorectal cancer cells as an in vitro model for personalizing cancer medicine. PLoS One. 2017;12:e0183074. doi: 10.1371/journal.pone.0183074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Atzori L, Poli G, Perra A. Hepatic stellate cell: a star cell in the liver. Int J Biochem Cell Biol. 2009;41:1639–1642. doi: 10.1016/j.biocel.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 85.Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Ann Rev Pathol. 2011;6:425–456. doi: 10.1146/annurev-pathol-011110-130246. [DOI] [PubMed] [Google Scholar]
- 86.Lee J, Choi B, No da Y, Lee G, Lee SR, Oh H, et al. A 3D alcoholic liver disease model on a chip. Integr Biol (Camb) 2016;8:302–308. doi: 10.1039/c5ib00298b. [DOI] [PubMed] [Google Scholar]
- 87.Pingitore P, Sasidharan K, Ekstrand M, Prill S, Lindén D, Human Romeo S. Multilineage 3D spheroids as a model of liver steatosis and fibrosis. Int J Mol Sci. 2019;20:E1629. doi: 10.3390/ijms20071629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Prior N, Inacio P, Huch M. Liver organoids: from basic research to therapeutic applications. Gut. 2019;68:2228–2237. doi: 10.1136/gutjnl-2019-319256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Takebe T, Zhang B, Radisic M. Synergistic engineering: organoids meet organs-on-a-chip. Cell Stem Cell. 2017;21:297–300. doi: 10.1016/j.stem.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 90.Williams DF. Challenges with the development of biomaterials for sustainable tissue engineering. Front Bioeng Biotechnol. 2019;7:127. doi: 10.3389/fbioe.2019.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zuppinger C. 3D cardiac cell culture: a critical review of current technologies and applications. Front Cardiovasc Med. 2019;6:87. doi: 10.3389/fcvm.2019.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kratochvil MJ, Seymour AJ, Li TL, Paşca SP, Kuo CJ, Heilshorn SC. Engineered materials for organoid systems. Nat Rev Mater. 2019;4:606–622. doi: 10.1038/s41578-019-0129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yanagi Y, Nakayama K, Taguchi T, Enosawa S, Tamura T, Yoshimaru K, et al. In vivo and ex vivo methods of growing a liver bud through tissue connection. Sci Rep. 2017;7:14085. doi: 10.1038/s41598-017-14542-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mitaka T. Reconstruction of hepatic organoid by hepatic stem cells. J Hepatobiliary Pancreat Surg. 2002;9:697–703. doi: 10.1007/s005340200096. [DOI] [PubMed] [Google Scholar]
- 95.Yin X, Mead BE, Safaee H, Langer R, Karp JM, Levy O. Engineering stem cell organoids. Cell Stem Cell. 2016;18:25–38. doi: 10.1016/j.stem.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jee JH, Lee DH, Ko J, Hahn S, Jeong SY, Kim HK, et al. Development of collagen-based 3D matrix for gastrointestinal tract-derived organoid culture. Stem Cells Int. 2019;2019:8472712. doi: 10.1155/2019/8472712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cruz-Acuña R, Quirós M, Farkas AE, Dedhia PH, Huang S, Siuda D, et al. Synthetic hydrogels for human intestinal organoid generation and colonic wound repair. Nat Cell Biol. 2017;19:1326–1335. doi: 10.1038/ncb3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Oksdath M, Perrin SL, Bardy C, Hilder EF, DeForest CA, Arrua RD, et al. Review: synthetic scaffolds to control the biochemical, mechanical, and geometrical environment of stem cell-derived brain organoids. APL Bioeng. 2018;2:041501. doi: 10.1063/1.5045124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Broutier L, Andersson-Rolf A, Hindley CJ, Boj SF, Clevers H, Koo BK, et al. Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat Protoc. 2016;11:1724–1743. doi: 10.1038/nprot.2016.097. [DOI] [PubMed] [Google Scholar]
- 100.Gjorevski N, Sachs N, Manfrin A, Giger S, Bragina ME, Ordóñez-Morán P, et al. Designer matrices for intestinal stem cell and organoid culture. Nature. 2016;539:560–564. doi: 10.1038/nature20168. [DOI] [PubMed] [Google Scholar]
- 101.Klotz BJ, Oosterhoff LA, Utomo L, Lim KS, Vallmajo-Martin Q, Clevers H, et al. A versatile biosynthetic hydrogel platform for engineering of tissue analogues. Adv Healthc Mater. 2019;8:e1900979. doi: 10.1002/adhm.201900979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ng SS, Saeb-Parsy K, Blackford SJI, Segal JM, Serra MP, Horcas-Lopez M, et al. Human iPS derived progenitors bioengineered into liver organoids using an inverted colloidal crystal poly (ethylene glycol) scaffold. Biomaterials. 2018;182:299–311. doi: 10.1016/j.biomaterials.2018.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Truskey GA. Human microphysiological systems and organoids as in vitro models for toxicological studies. Front Public Health. 2018;6:185. doi: 10.3389/fpubh.2018.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bhatia SN, Ingber DE. Microfluidic organs-on-chips. Nat Biotechnol. 2014;32:760–772. doi: 10.1038/nbt.2989. [DOI] [PubMed] [Google Scholar]
- 105.Schepers A, Li C, Chhabra A, Seney BT, Bhatia S. Engineering a perfusable 3D human liver platform from iPS cells. Lab Chip. 2016;16:2644–2653. doi: 10.1039/c6lc00598e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang Y, Wang H, Deng P, Chen W, Guo Y, Tao T, et al. In situ differentiation and generation of functional liver organoids from human iPSCs in a 3D perfusable chip system. Lab Chip. 2018;18:3606–3616. doi: 10.1039/c8lc00869h. [DOI] [PubMed] [Google Scholar]
- 107.Wang Y, Takeishi K, Li Z, Cervantes-Alvarez E, Collin de l’Hortet A, Guzman-Lepe J, et al. Microenvironment of a tumor-organoid system enhances hepatocellular carcinoma malignancy-related hallmarks. Organogenesis. 2017;13:83–94. doi: 10.1080/15476278.2017.1322243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nuciforo S, Fofana I, Matter MS, Blumer T, Calabrese D, Boldanova T, et al. Organoid models of human liver cancers derived from tumor needle biopsies. Cell Rep. 2018;24:1363–1376. doi: 10.1016/j.celrep.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Buzzelli JN, Ouaret D, Brown G, Allen PD, Muschel RJ. Colorectal cancer liver metastases organoids retain characteristics of original tumor and acquire chemotherapy resistance. Stem Cell Res. 2018;27:109–120. doi: 10.1016/j.scr.2018.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Roper J, Tammela T, Cetinbas NM, Akkad A, Roghanian A, Rickelt S, et al. In vivo genome editing and organoid transplantation models of colorectal cancer and metastasis. Nat Biotechnol. 2017;35:569–576. doi: 10.1038/nbt.3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pinzani M. Pathophysiology of liver fibrosis. Dig Dis. 2015;33:492–497. doi: 10.1159/000374096. [DOI] [PubMed] [Google Scholar]
- 112.Leite SB, Roosens T, El Taghdouini A, Mannaerts I, Smout AJ, Najimi M, et al. Novel human hepatic organoid model enables testing of drug-induced liver fibrosis in vitro. Biomaterials. 2016;78:1–10. doi: 10.1016/j.biomaterials.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 113.Mazza G, Al-Akkad W, Rombouts K. Engineering in vitro models of hepatofibrogenesis. Adv Drug Deliv Rev. 2017;121:147–157. doi: 10.1016/j.addr.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 114.Nantasanti S, Spee B, Kruitwagen HS, Chen C, Geijsen N, Oosterhoff LA, et al. Disease modeling and gene therapy of copper storage disease in canine hepatic organoids. Stem Cell Reports. 2015;5:895–907. doi: 10.1016/j.stemcr.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stoller JK, Aboussouan LS. α1-antitrypsin deficiency. Lancet. 2005;365:2225–2236. doi: 10.1016/S0140-6736(05)66781-5. [DOI] [PubMed] [Google Scholar]
- 116.van De Sluis B, Rothuizen J, Pearson PL, van Oost BA, Wijmenga C. Identification of a new copper metabolism gene by positional cloning in a purebred dog population. Hum Mol Genet. 2002;11:165–173. doi: 10.1093/hmg/11.2.165. [DOI] [PubMed] [Google Scholar]
- 117.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263–2273. doi: 10.1001/jama.2015.5370. [DOI] [PubMed] [Google Scholar]
- 118.Kruitwagen HS, Oosterhoff LA, Vernooij I, Schrall IM, van Wolferen ME, Bannink F, et al. Long-term adult feline liver organoid cultures for disease modeling of hepatic steatosis. Stem Cell Reports. 2017;8:822–830. doi: 10.1016/j.stemcr.2017.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Protzer U, Maini MK, Knolle PA. Living in the liver: hepatic infections. Nat Rev Immunol. 2012;12:201–213. doi: 10.1038/nri3169. [DOI] [PubMed] [Google Scholar]
- 120.Dutta D, Heo I, Clevers H. Disease modeling in stem cell-derived 3D organoid systems. Trends Mol Med. 2017;23:393–410. doi: 10.1016/j.molmed.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 121.Hakim MS, Chen S, Ding S, Yin Y, Ikram A, Ma XX, et al. Basal interferon signaling and therapeutic use of interferons in controlling rotavirus infection in human intestinal cells and organoids. Sci Rep. 2018;8:8341. doi: 10.1038/s41598-018-26784-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ettayebi K, Crawford SE, Murakami K, Broughman JR, Karandikar U, Tenge VR, et al. Replication of human noroviruses in stem cell-derived human enteroids. Science. 2016;353:1387–1393. doi: 10.1126/science.aaf5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Drummond CG, Bolock AM, Ma C, Luke CJ, Good M, Coyne CB. Enteroviruses infect human enteroids and induce antiviral signaling in a cell lineage-specific manner. Proc Natl Acad Sci U S A. 2017;114:1672–1677. doi: 10.1073/pnas.1617363114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yin Y, Bijvelds M, Dang W, Xu L, van der Eijk AA, Knipping K, et al. Modeling rotavirus infection and antiviral therapy using primary intestinal organoids. Antivir Res. 2015;123:120–131. doi: 10.1016/j.antiviral.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 125.Baktash Y, Madhav A, Coller KE, Randall G. Single particle imaging of polarized hepatoma organoids upon hepatitis C virus infection reveals an ordered and sequential entry process. Cell Host Microbe. 2018;23:382–394. doi: 10.1016/j.chom.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sakiyama R, Blau BJ, Miki T. Clinical translation of bioartificial liver support systems with human pluripotent stem cell-derived hepatic cells. World J Gastroenterol. 2017;23:1974–1979. doi: 10.3748/wjg.v23.i11.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Iansante V, Mitry RR, Filippi C, Fitzpatrick E, Dhawan A. Human hepatocyte transplantation for liver disease: current status and future perspectives. Pediatr Res. 2018;83:232–240. doi: 10.1038/pr.2017.284. [DOI] [PubMed] [Google Scholar]
- 128.Xie B, Sun D, Du Y, Jia J, Sun S, Xu J, et al. A two-step lineage reprogramming strategy to generate functionally competent human hepatocytes from fibroblasts. Cell Res. 2019;29:696–710. doi: 10.1038/s41422-019-0196-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hu C, Li L. In vitro culture of isolated primary hepatocytes and stem cell-derived hepatocyte-like cells for liver regeneration. Protein Cell. 2015;6:562–574. doi: 10.1007/s13238-015-0180-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tsuda Y, Kikuchi A, Yamato M, Chen G, Okano T. Heterotypic cell interactions on a dually patterned surface. Biochem Biophys Res Commun. 2006;348:937–944. doi: 10.1016/j.bbrc.2006.07.138. [DOI] [PubMed] [Google Scholar]
- 131.Zhu L, Xia H, Wang Z, Fong EL, Fan J, Tong WH, et al. A vertical-flow bioreactor array compacts hepatocytes for enhanced polarity and functions. Lab Chip. 2016;16:3898–3908. doi: 10.1039/c6lc00811a. [DOI] [PubMed] [Google Scholar]
- 132.Cohen S, Baño MC, Cima LG, Allcock HR, Vacanti JP, Vacanti CA, et al. Design of synthetic polymeric structures for cell transplantation and tissue engineering. Clin Mater. 1993;13:3–10. doi: 10.1016/0267-6605(93)90082-i. [DOI] [PubMed] [Google Scholar]
- 133.Lund AW, Yener B, Stegemann JP, Plopper GE. The natural and engineered 3D microenvironment as a regulatory cue during stem cell fate determination. Tissue Eng Part B Rev. 2009;15:371–380. doi: 10.1089/ten.teb.2009.0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kaviani M, Azarpira N. Insight into microenvironment remodeling in pancreatic endocrine tissue engineering: biological and biomaterial approaches. Tissue Eng Regen Med. 2016;13:475–484. doi: 10.1007/s13770-016-0014-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Miri AK, Khalilpour A, Cecen B, Maharjan S, Shin SR, Khademhosseini A. Multiscale bioprinting of vascularized models. Biomaterials. 2019;198:204–216. doi: 10.1016/j.biomaterials.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Koike H, Iwasawa K, Ouchi R, Maezawa M, Giesbrecht K, Saiki N, et al. Modelling human hepato-biliary-pancreatic organogenesis from the foregut-midgut boundary. Nature. 2019;574:112–116. doi: 10.1038/s41586-019-1598-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Aw Yong KM, Li Z, Merajver SD, Fu J. Tracking the tumor invasion front using long-term fluidic tumoroid culture. Sci Rep. 2017;7:10784. doi: 10.1038/s41598-017-10874-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lee SW, Hong S, Jung B, Jeong SY, Byeon JH, Jeong GS, et al. In vitro lung cancer multicellular tumor spheroid formation using a microfluidic device. Biotechnol Bioeng. 2019;116:3041–3052. doi: 10.1002/bit.27114. [DOI] [PubMed] [Google Scholar]
- 139.Lee SW, Kwak HS, Kang MH, Park YY, Jeong GS. Fibroblast-associated tumour microenvironment induces vascular structure-networked tumouroid. Sci Rep. 2018;8:2365. doi: 10.1038/s41598-018-20886-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Montanez-Sauri SI, Sung KE, Berthier E, Beebe DJ. Enabling screening in 3D microenvironments: probing matrix and stromal effects on the morphology and proliferation of T47D breast carcinoma cells. Integr Biol (Camb) 2013;5:631–640. doi: 10.1039/c3ib20225a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Abaci HE, Guo Z, Doucet Y, Jacków J, Christiano A. Next generation human skin constructs as advanced tools for drug development. Exp Biol Med (Maywood) 2017;242:1657–1668. doi: 10.1177/1535370217712690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pamies D, Hartung T, Hogberg HT. Biological and medical applications of a brain-on-a-chip. Exp Biol Med (Maywood) 2014;239:1096–1107. doi: 10.1177/1535370214537738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lehmann R, Lee CM, Shugart EC, Benedetti M, Charo RA, Gartner Z, et al. Human organoids: a new dimension in cell biology. Mol Biol Cell. 2019;30:1129–1137. doi: 10.1091/mbc.E19-03-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Skylar-Scott MA, Uzel SGM, Nam LL, Ahrens JH, Truby RL, Damaraju S, et al. Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels. Sci Adv. 2019;5:eaaw2459. doi: 10.1126/sciadv.aaw2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Candiello J, Grandhi TSP, Goh SK, Vaidya V, Lemmon-Kishi M, Eliato KR, et al. 3D heterogeneous islet organoid generation from human embryonic stem cells using a novel engineered hydrogel platform. Biomaterials. 2018;177:27–39. doi: 10.1016/j.biomaterials.2018.05.031. [DOI] [PubMed] [Google Scholar]
- 146.Lee HK, Velazquez Sanchez C, Chen M, Morin PJ, Wells JM, Hanlon EB, et al. Three dimensional human neuro-spheroid model of alzheimer’s disease based on differentiated induced pluripotent stem cells. PLoS One. 2016;11:e0163072. doi: 10.1371/journal.pone.0163072. [DOI] [PMC free article] [PubMed] [Google Scholar]