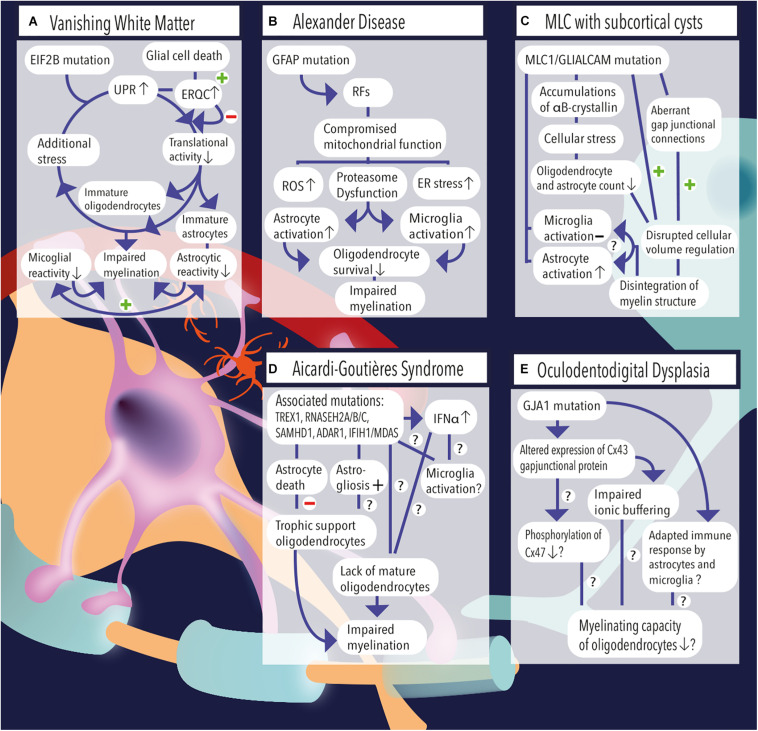
FIGURE 1.
(A) Vanishing white matter is caused by recessive mutations in the EIF2B gene. Induction of the unfolded protein response (UPR) causes lowering of the cellular translational activity and results in glial cell immaturity (e.g., astrocytes and oligodendrocytes). Additional stress (febrile infections or minor head trauma) further induces the cycle of cellular stress. Rescue mechanisms such as endoplasmic reticulum quality control (ERQC) are activated to escape a vicious UPR cycle and promote glial cell death if cellular damage exceeds the threshold for recovery. Vanishing white matter specimens characteristically show reduced astrocytic and microglial reactivity. Together, the lack of mature astrocytes and oligodendrocytes as well as the absence glial cell activation impairs the process of myelination. (B) Overexpression of GFAP due to homozygous mutations in the GFAP gene causes the formation of Rosenthal fibers (RFs) in the cytosol of astrocytes. Astrocytic pathology leads to an impaired respiratory chain complex (i.e., impaired mitochondrial function), which is indicated by increased levels of reactive oxygen species (ROS), endoplasmic reticulum (ER) stress, and proteasome dysfunction as pointed out by activation of kinase pathways. Pathological hallmarks of Alexander disease are prominent activation of astrocytes and microglia. It is hypothesized that sustained activation of both cell types has a detrimental effect on oligodendrocyte survival and contribute to a lack or loss of myelin. (C) MLC1 or GLIALCAM mutations are causally related to the development of megalencephalic leukoencephalopathy (MLC) with subcortical cysts. Accumulations of αB-crystallin have been observed in the cytosol of MLC astrocytes. The resulting cellular stress might relate to the observed reduction in the number (#) of oligodendrocytes and astrocytes. Although microglial activation is absent, MLC specimens demonstrate substantial astrogliosis, which usually are physiologically coupled events. Dysregulation of fluid excesses after neuronal depolarizations (i.e., disrupted cellular volume regulation) results in disintegration of the myelin layers and presence of intramyelinic vacuoles. (D) Aicardi–Goutières syndrome’ associated mutations include TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR1, and IFIH1/MDAS, which result in an increased and dysregulated production of interferon-alpha (IFNα), which might be toxic to oligodendrocytes and might induce sustained activation of microglia. Furthermore, AGS mutations have been related to increased astrogliosis and astrocyte death. As oligodendrocytes are highly dependent on trophic support by neighboring astrocytes, their survival rate is severely affected and myelinating capacity is limited. Yet, it is unclear whether Aicardi–Goutières-associated mutations might (also) have an effect on microglial activation or oligodendrocyte maturation directly. (E) A mutation in the GJA1 gene, which is associated with oculodentodigital dysplasia, causes altered expression of the gap junctional hemichannel Cx43, which is abundantly expressed in astrocyte–astrocyte and astrocyte–oligodendrocyte contacts. A direct consequence of altered Cx43 expression might be a phosphorylation defect of Cx47 on the oligodendrocyte’ membrane, which in turn negatively affects their myelinating capacity. In addition, impaired ionic buffering of K+ and Na+ ions might affect oligodendrocyte homeostasis and affect the process of myelination. In addition, adaptations in the immunological response of astrocytes and microglia have been observed and related to altered expression of Cx43.