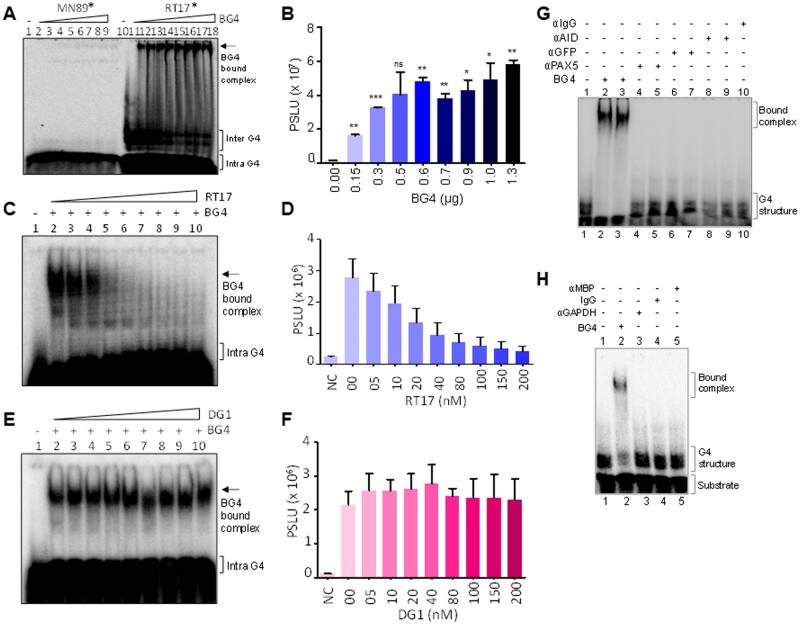
Figure 1.
Evaluation of specificity of BG4 antibody towards G-quadruplex DNA. (A) Gel image showing BG4 binding to G4 DNA. Radiolabelled Hif1α oligomeric substrate, RT17* (lanes 10–18), was incubated in KCl at 37°C, followed by incubation with no or increasing concentrations of BG4 (0.15, 0.3, 0.5, 0.6, 0.7, 0.9, 1.0 and 1.3 µg). Bound DNA was then electrophoresed on a 6% native PAGE and indicated using an arrow. Complementary C-rich sequence, MN89*, fails to show any binding (lanes 1–9). Asterisk represents radiolabelled substrate. (B) Bar diagram showing increase in formation of bound complex for RT17 with an increase in BG4 concentration (P < 0.05). (C) Competition assay using radiolabelled RT17, in presence of increasing concentration of unlabelled (cold) RT17, as the specific competitor. Briefly, radiolabelled RT17* was allowed to form G4 structure at 37°C, followed by incubation with BG4. Simultaneously, unlabelled RT17 (0, 05, 10, 20, 40, 80, 100, 150, 200 nM) was added to the reaction mixture, incubated and the products were resolved on a 6% native PAGE. Bound complex is indicated by an arrow. (D) Bar diagram showing reduction in bound complex when incubated with increasing concentration of unlabelled oligomer RT17 (P < 0.05), indicating specificity of antibody towards G4 DNA. (E) Competition assay to evaluate BG4 binding to radiolabelled RT17* in presence of cold random oligomeric DNA, DG1. Radiolabelled RT17* was allowed to form G4 at 37°C, followed by BG4 incubation. Unlabelled DG1 (0, 05, 10, 20, 40, 80, 100, 150, 200 nM) was added to reactions, and the products were resolved on a 6% native PAGE. (F) Bar diagram showing BG4 bound complex (P < 0.05), when cold random DNA substrate was added to the mixture confirming the specificity of antibody towards G4 DNA. (G, H) Gel images showing specificity of BG4. Various antibodies (200 ng) were incubated with RT17 in the same conditions as that of BG4, and the products were resolved on 6% native PAGE. Bound complex and substrate have been indicated in the gel images.