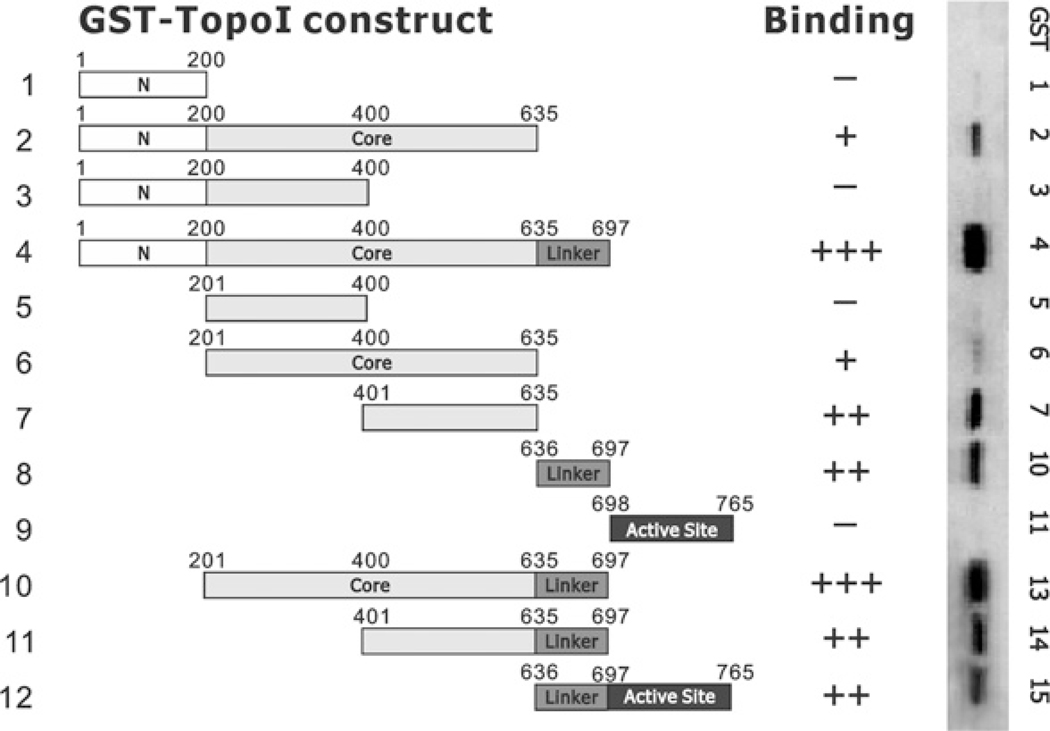
Figure 1. Binding of NKX3.1 to GST fusion peptides of topoisomerase I.
GST–topoisomerase I (Top1) fusion constructs (peptides 1–12) were made as described in the Experimental section. Fusion proteins were expressed in BL21 cells and purified with glutathione beads. For the GST pull-down assay, GST-fusion proteins were bound to glutathione beads, washed and incubated with 500 μg of LNCaP cell lysates overnight. After washing five times with PBS containing 0.2% Triton-X 100, beads were eluted with 2× SDS loading buffer at 99°C for 5 min. Eluted proteins were fractionated by SDS/PAGE (4–20% gels) and transferred on to nitrocellulose membranes. NKX3.1 was Western blotted with an anti-NKX3.1 antibody. the Western blot results are shown in the right-hand panel with the intensity of the signals graded as − to +++.