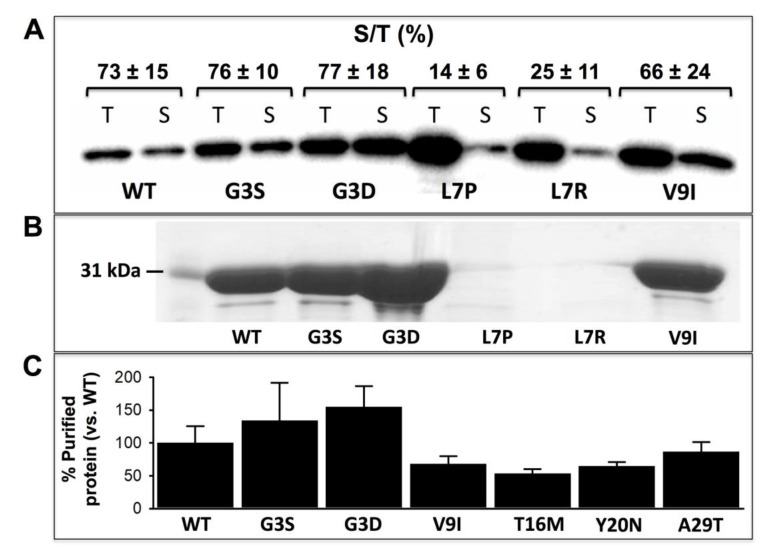
Figure 2.
Expression and solubility of NQO1 variants in E. coli cells. (A) Western-blot analysis of NQO1 content upon expression in E. coli cells. Cells were sonicated to obtain total extracts (T), while soluble extracts (S) were obtained upon centrifugation at 21,000× g for 20 min at 4 °C. Samples were denatured with Laemmli buffer and submitted for Western-blot analysis using anti-NQO1 antibody, F-8 antibody (Santa Cruz Biotechnology). The Western-blot is representative from three different purifications. The fraction of the total protein found in the soluble extract upon densitometric analysis (S/T, as %) is indicated (as mean ± s.d. from three independent experiments). (B) Purified NQO1 proteins from three different purifications were concentrated ~10-fold and samples analyzed by SDS-PAGE. Note that during purification and storage, the remaining soluble protein of L7P and L7R was negligible. (C) Yield in NQO1 protein variants after immobilized-metal affinity chromatography (IMAC) purification. Data were the mean ± s.d. from 3–4 different purifications for each NQO1 variant. Wild-type (WT) levels were 1.45 ± 0.38 mg·L−1 of culture and used to normalize yields.