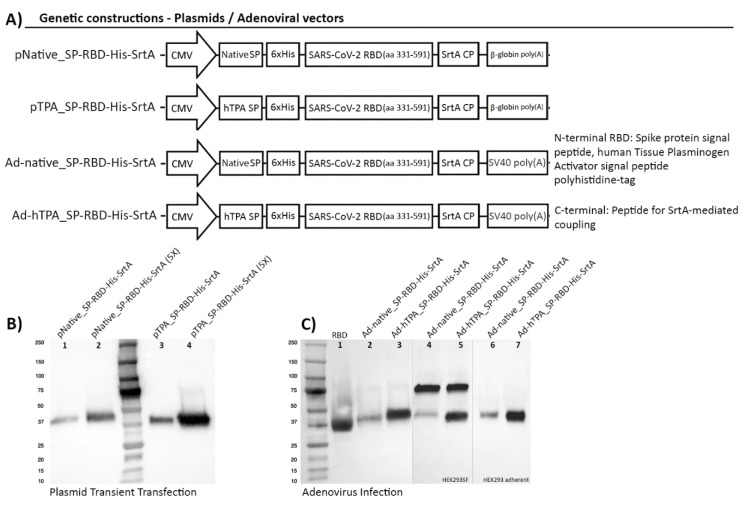
Figure 1.
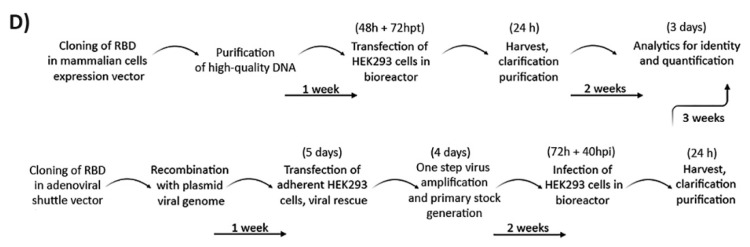
Genetic construction design of SARS-CoV-2 RBD expression cassettes and detection of the recombinant protein by transient transfection and adenoviral infection of HEK293SF cells. (A) Schematic representation of constructions encoding the RBD preceded by the native Spike signal peptide (SP) or the human Plasminogen Tissue Activator (hTPA) secretion signal, followed by a polyhistidine tag (6xHis). The coding sequence for the bacterial Sortase A coupling peptide (SrtA, CP) was added at the C-terminal of the protein. The diagram shows the regulatory regions that control the expression of RBD: (i) a fragment of the human cytomegalovirus enhancer/promoter (hCMV) was used in all the constructions, (ii) the rabbit β-globin polyA signal was used in the plasmid constructions for transient transfection, and the SV40 polyA fragment in the adenoviral constructions. (B) Detection by Western Blot of RBD expression in 25 mL of culture supernatant 72 h post-transfection (hpt) in shake flasks using HyCell TransFx-H medium. An anti His-tag monoclonal antibody was used. The secreted antigen is detected with an apparent molecular weight of approximately 38 kDa. Lanes 1, 2 correspond to the protein expressed under the native Spike signal peptide and lanes 3, 4 to secretion driven by the hTPA secretion signal. In lanes 2 and 4, (5X) corresponds to a 5-fold concentration of culture supernatant. Equal amounts of total proteins (3.6 ug for lanes 1 and 3 and 12.6 ug for lanes 2 and 4) were loaded onto the gel, enabling to confirm that higher RBD secretion levels were obtained with the use of the hTPA signal peptide. (C) Detection by Western blot of RBD expression mediated by adenoviral infection of HEK293SF cells. The anti His-tag monoclonal antibody (lanes 1–3) and a polyclonal anti-SARS-CoV-2 Spike antibody (4–7) were used. The protein was similarly expressed with a molecular weight of about 38 kDa. The polyclonal antibody used showed non-specific binding to one protein identified as lactoferrin, present in the culture medium at a molecular weight of approximately 75 kDa. Lanes 2, 4and 3, 5 show the expression of RBD using the native signal peptide and hTPA, respectively, at the time of harvest, 40 h post-infection, as recognized with each antibody. Lanes 6 and 7 correspond to detection of RBD under the two signal peptides in HEK293 adherent cells at the time of virus rescue (note no presence of lactoferrin at 75 kDa when using DMEM + 10%FBS). Lane 1 in (C) corresponds to RBD used as positive control, as referenced in Materials and Methods. (D) Diagram representing the workflow and timelines for the accelerated production of RBD following the two methods described.