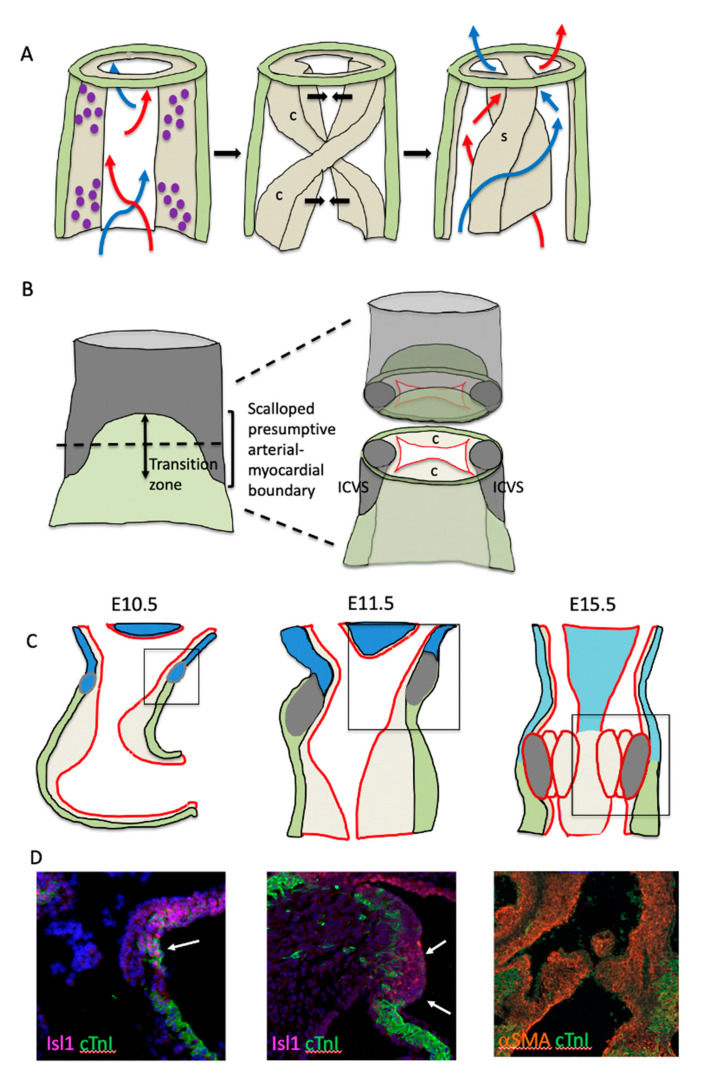
Figure 2.
Early outflow tract development. (A) Model representing how flow of blood through the unseptated outflow tract may lead to formation of the spiralling outflow septum. Vorticial flow of blood through the outflow tract as the cushions are beginning to cellularise leads to aggregation of these cells (purple dots) in columns that prefigure the spiralling cushions (c). This positions and/or stabilises the cellularising cushions within the circumference of the outflow tract. The ensuing fusion of these cushions leads to formation of a spiralling septum (s) that places the root of the aorta to the left of the pulmonary trunk. (B) The boundary between myocardial and non-myocardial fate is first apparent at 10.5 in the mouse as the transition zone. The cells in this region are transitioning from a progenitor (grey) to a cardiomyocyte (green) fate. However, not all the undifferentiated SHF cells within this boundary region become cardiomyocytes. Two tongues of undifferentiated SHF cells can be seen to extend laterally into the myocardial outflow tract and these form the intercalated valve swellings (ICVS) that are the primordia of the posterior (non-coronary) and anterior intercalated leaflets of the aortic and pulmonary valves. (C,D) The myocardial-non-myocardial boundary is labelled by Isl1 (pink) and cTnI (green) at E10.5, with the cells in the transition zone labelled by both markers (arrow). By E11.5 the ICVS can clearly be seen in the outflow wall, as it continues to express Isl1 (arrows). By E15.5 the myocardial (green)-SMC (red) boundary at the level of the valve complex is well defined.