Figure 1.
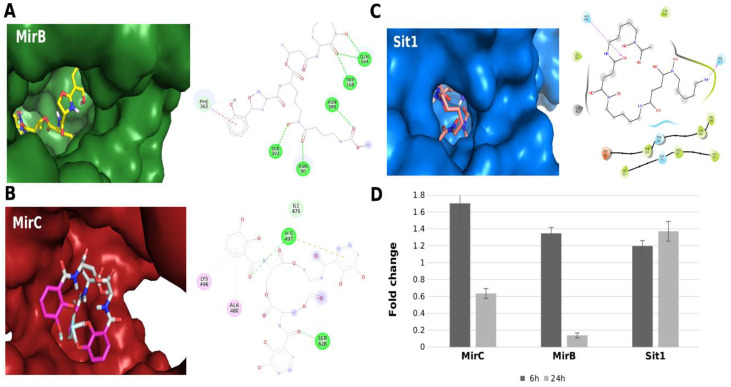
Interactions between three-dimensional models and siderophores. (A) Interaction between MirB and carboxymycobactin. The left panel shows the coupling of siderophore in the MirB pocket (green). In the right panel, it is observed that the interaction is maintained by conventional hydrogen bonds (green), carbon–hydrogen bonds (light blue), and pi–pi stacked interaction (pink) between the aromatic rings of carboxymycobactin and PHE362. (B) Interaction between MirC and enterobactin. The left panel shows the coupling of the siderophore in the MirC pocket (red). In the right panel, the interaction is maintained by conventional hydrogen (green) and carbon–hydrogen (light blue) bonds, in addition to the pi–alkyl (pink) interaction between the aromatic enterobactin ring and the amino acids LYS496 and ALA480 and the pi–cation interaction (yellow) between the aromatic ring and the HIS497. (C) Interaction between Sit1 and ferrioxamine B. The left panel shows the coupling of the siderophore in the Sit1 pocket (blue). In the right panel, it is observed that the ligand ferrioxamine B is maintained mainly by hydrophobic affinity (green) to the pocket of Sit1. (D) Levels of transcripts from siderophore transporter genes accessed by RT-qPCR after cultivation of P. brasiliensis in Fe deprivation or in the presence of FOB for 6 h and 24 h. For a list of the primers used in the experiments, see Supplementary Table S3. Additional information on this transcriptional analysis is available in the Supplementary Materials.