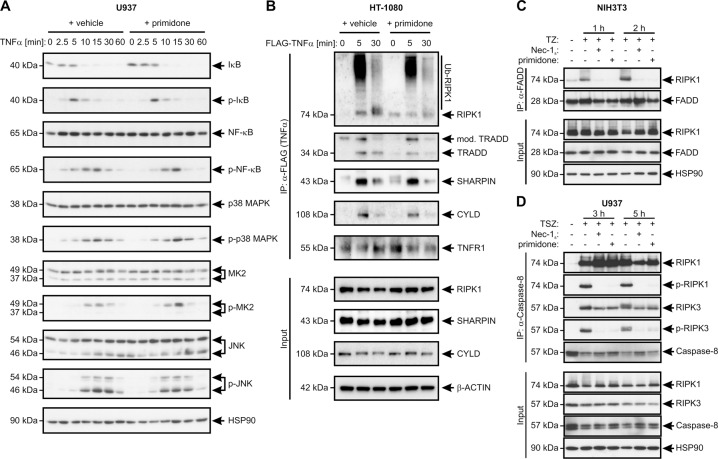
Fig. 3. Primidone does not intervene in canonical NF-κB signaling after TNFR1 activation.
A Human U937 cells were stimulated with 100 ng/ml TNFα in the absence or presence of 1 mM primidone for the indicated durations. Primidone was added 30 min before the addition of TNFα. Western blotting analysis of the cell lysates was performed using the indicated antibodies. B TNFα-induced complex I immunoprecipitation (IP) of human HT-1080 cells treated in the absence or presence of 1 mM primidone with 1 μg/ml FLAG-tagged TNFα (FLAG-TNFα) for the indicated durations, followed by anti-FLAG IP and western blotting analysis using the indicated antibodies. The results presented in A, B indicate that the biological effect of primidone is manifested in death-signaling complex II. C In murine cells such as NIH3T3 cells, primidone prevented the assembly of complex II. The macromolecular association of FADD, RIPK1, and RIPK3, which served as a platform for subsequent cell death was directly affected by primidone, blocking the activation (phosphorylation) of RIPK1. NIH3T3 cells were treated with 100 ng/ml TNFα + 25 μM zVAD (TZ) in the absence or presence of the RIPK1 inhibitor Nec-1s and 1 mM primidone, respectively, for the indicated durations. Nec-1s and primidone were added each 30 min before the addition of TNFα. TZ-induced complex II was immunoprecipitated with α-FADD antibody from cell lysates. Lysates pre-IP (Input) were also analyzed by western blotting using the indicated antibodies. D In human cells such as U937 cells, primidone did not prevent the assembly of the cytosolic death-inducing signaling complex II, but affected RIPK1 and RIPK3 activation, preventing TNFα-induced cell death. U937 cells were treated with 100 ng/ml TNFα + 1 μM SMAC mimetic SM-164 + 25 μM zVAD (TSZ) in the absence or presence of the RIPK1 inhibitor Nec-1s and 1 mM primidone, respectively. Nec-1s and primidone were each added 30 min before the addition of TNFα. The TSZ-induced complex II was immunoprecipitated with anti-caspase-8 antibody from the cell lysates. Lysates pre-IP (Input) were also analyzed by western blotting using the indicated antibodies. A–D Each blot was stripped and re-probed with an antibody against HSP90 or β-Actin (β-ACTIN) as the loading control. For each, one representative cropped blot of three independent experiments is shown.