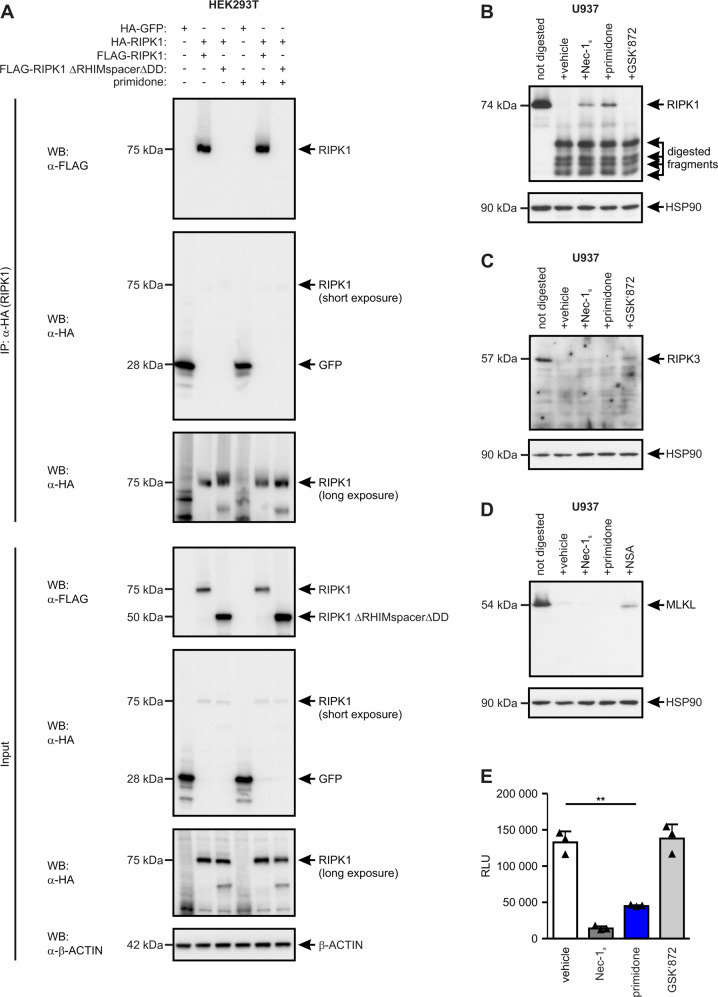
Fig. 4. Primidone operates as a kinase inhibitor of RIPK1.
A The possibility that the protective effect of primidone in RIPK1-mediated cell death is due to avoiding RHIM- and DD-mediated dimerization and/or oligomerization of RIPK1 was ruled out via expression experiments with tagged recombinant RIPK1 proteins. B–D The Drug Affinity Responsive Target Stability (DARTS) assay identified RIPK1 as a target of primidone. U937 cells were lysed, cleared, and incubated for 20 min at room temperature in the presence of the indicated molecules. Subsequently, protein lysates were digested for 5 min using thermolysin. B Increased resistance to proteolysis of RIPK1 was detected upon binding of primidone as well as Nec-1s by western blotting using anti-RIPK1 antibody. Nonselective interference of small-molecules with this assay was excluded by the addition of GSK'872, a selective inhibitor of the structurally related RIPK3 that does not affect digestion of RIPK1. Simultaneous proteolysis of RIPK3 and MLKL in this setting by primidone or Nec-1s was excluded by western blots using anti-RIPK3 (C) and anti-MLKL (D) antibody, respectively, in which the suitability of the DARTS assay was confirmed by the addition of GSK’872 (C) and NSA (D) as selective inhibitors of RIPK3 and human MLKL, respectively [40, 56]. All blots were stripped and re-probed with an antibody against HSP90 as the loading control. One representative cropped blot of three independent experiments each is shown. E The ADP-Glo™ Kinase Assay used to measure the kinase activity of recombinant human RIPK1 (aa 1-327) revealed that primidone is an effective kinase inhibitor of RIPK1 (RLU = relative light units). In parallel, Nec-1s (a confirmed kinase inhibitor of RIPK1) and GSK’872 (a specific kinase inhibitor of RIPK3) were integrated in the assay as internal controls. The graph shows the mean ± SD of three independent experiments.