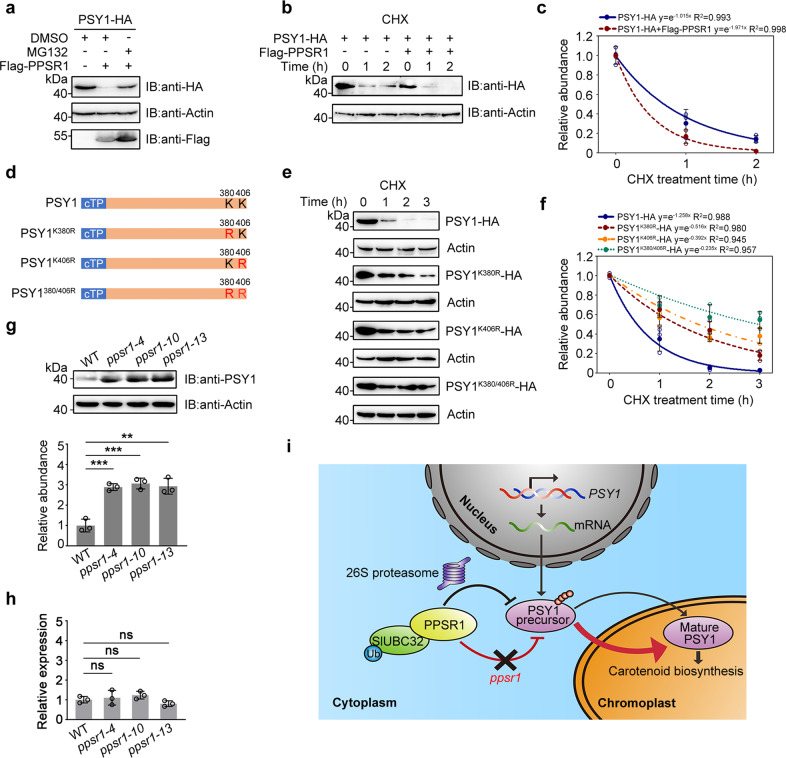
Fig. 6. PPSR1 modulates PSY1 protein level via ubiquitination.
a Stability assay of PSY1. The PSY1-HA fusion protein was co-expressed with Flag-PPSR1 in tobacco (Nicotiana benthamiana) leaves. The total proteins were extracted and submitted to immunoblot using anti-HA and anti-Flag antibodies, respectively. The N. benthamiana actin was used as the loading control. IB, immunoblot. b Degradation rate assays of PSY1 under the action of PPSR1. The PSY1-HA and Flag-PPSR1 fusion proteins were co-expressed in tobacco leaves. The leaves were treated with translation inhibitor cycloheximide (CHX), and the total proteins were extracted for immunoblotting with anti-HA antibody at an indicated time point after treatment. c Quantification of protein levels in (b) by ImageJ. d Diagram showing PSY1 with ubiquitination site mutations. K, lysine; R, arginine. e Degradation rate assays of PSY1 and its mutated forms. The PSY1 and its mutated forms expressed in tobacco leaves were treated with CHX and submitted to immunoblot as described in (b). f Quantification of protein levels in (e) by ImageJ. g Expression of PSY1 protein in fruit of wild-type (WT) and ppsr1 mutants. Total protein was extracted and submitted to immunoblot using anti-PSY1 antibody (upper panel). Equal loading was confirmed by an anti-actin antibody. The protein levels were quantified by ImageJ (lower panel). h Gene expression of PSY1 in fruit of WT and ppsr1 mutants. Total RNA was isolated and submitted to quantitative real-time PCR. The ACTIN gene was used as the internal control. For (c), (f), (g), and (h), error bars represent the means ± standard deviation (SD) of three independent experiments. The circles indicate individual data points. Asterisks indicate significant differences (**P < 0.01, ***P < 0.001; two-tailed Student’s t-test). i The working model for PPSR1-mediated post-translational regulation of PSY1. PPSR1 directly interacts with SlUBC32 and mediates degradation of PSY1 precursor, which is nucleus-encoded and synthesized in the cytosol, via 26S proteasome. In the absence of PPSR1, more PSY1 precursor was transported into the plastid, leading to the accumulation of PSY1 protein. The increased PSY1 protein accelerates the biosynthesis of carotenoid in the plastid.