FIGURE 2.
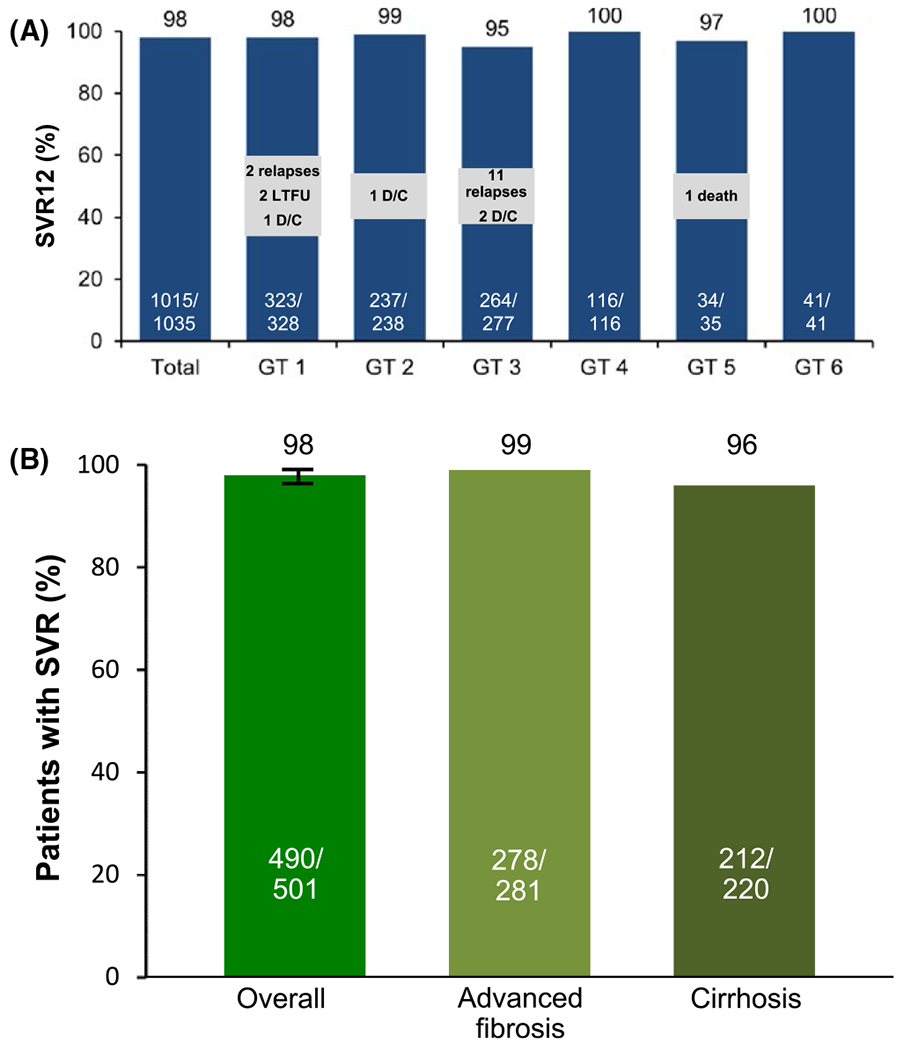
(A) SOF/VEL SVR rates for 12 weeks across all genotypes (ASTRAL-1-2-3).10,11 These studies encompassed a broad range of HCV populations, including HCV genotypes 1-6. The overall SVR rate in more than 1000 patients was high, with 98% achieving SVR12. AE, adverse event; D/C, discontinuation; LTFU, lost to followup; SAE, serious adverse event. (B) Sustained virological response rates overall and by cirrhosis or advanced fibrosis status.13 (ASTRAL-1-2-3). SVR12 was achieved by 98% of patients (490/501; 95% CI, 96-99). Patients with cirrhosis had an SVR12 rate of 96% (212/220), and those with advanced fibrosis had an SVR12 rate of 99% (278/281)
