Abstract
PURPOSE
It is estimated that 50%-80% of patients with pediatric cancer in sub-Saharan Africa present at an advanced stage. Delays can occur at any time during the care-seeking process from symptom onset to treatment initiation. Referral delay, the time from first presentation at a health facility to oncologist evaluation, is a key component of total delay that has not been evaluated in sub-Saharan Africa.
METHODS
Over a 3-month period, caregivers of children diagnosed with cancer at a regional cancer center (Bugando Medical Centre [BMC]) in Tanzania were consecutively surveyed to determine the number and type of health facilities visited before presentation, interventions received, and transportation used to reach each facility.
RESULTS
Forty-nine caregivers were consented and included in the review. A total of 124 facilities were visited before BMC, with 31% of visits (n = 38) resulting in a referral. The median referral delay was 89 days (mean, 122 days), with a median of two facilities (mean, 2.5 facilities) visited before presentation to BMC. Visiting a traditional healer first significantly increased the time taken to reach BMC compared with starting at a health center/dispensary (103 v 236 days; P = .02). Facility visits in which a patient received a referral to a higher-level facility led to significantly decreased time to reach BMC (P < .0001). Only 36% of visits to district hospitals and 20.6% of visits to health centers/dispensaries yielded a referral, however.
CONCLUSION
The majority of patients were delayed during the referral process, but receipt of a referral to a higher-level facility significantly shortened delay time. Referral delay for pediatric patients with cancer could be decreased by raising awareness of cancer and strengthening the referral process from lower-level to higher-level facilities.
INTRODUCTION
An estimated 400,000 children worldwide are diagnosed with cancer each year.1 While survival rates for pediatric cancer in high-income countries are > 80%, survival rates are often < 25% in low- and middle-income countries (LMICs), where 90% of all new patients will be diagnosed.2-5 This means that the majority of pediatric cancer deaths occur in LMICs each year. The causes of this global disparity in survival among patients with pediatric cancer are multifactorial, but delayed presentation to treatment is a major contributing factor.6-10 In sub-Saharan Africa (SSA), delayed presentation has been recognized as an important barrier to effective care since cancer care efforts began in the region in the 1960s.11-15
CONTEXT
Key Objective
Given that a majority of patients with pediatric cancer in sub-Saharan Africa present at an advanced stage, to what extent do referral delays affect overall patient delay?
Knowledge Generated
Median referral delay to a cancer treatment center in Tanzania was 89 days, and patients visited a median of two health facilities before reaching the cancer center. Facility visits where a patient was given a referral to a higher-level facility significantly decreased time to cancer care.
Relevance
Raising awareness about pediatric cancer and strengthening the referral process from lower-level health facilities to cancer care could help to downstage pediatric cancer.
A multisite study in SSA estimated that 50%-80% of patients with pediatric cancer present at an advanced stage because of presentation delays.16 Late presentation worsens treatment outcomes17,18 and can cause families to incur both high direct costs (eg, transportation) and indirect costs (eg, missed work).19 These costs may actually reduce the cost effectiveness of treatment.20,21 To date, there has been little progress toward addressing presentation delays and downstaging cancer in LMICs.17,22-25
Bugando Medical Centre (BMC) is one of three specialist referral centers that treat cancer in Tanzania, serving a catchment area of 15 million people in northern Tanzania. Studies of various adult malignancies at BMC have found that similar to the rest of SSA, a majority (69%-97%) of patients with cancer present at late stages.26-29
This study focused on referral delay, defined as the time between first visit to a medical facility and evaluation by an oncologist, and its role in overall presentation delay for patients with pediatric cancer at BMC. To our knowledge, this study is the first to explore in-depth the referral delay for patients with pediatric cancer in SSA.
METHODS
Data Collection
All caregivers of children diagnosed with cancer < 18 years of age who presented to BMC over a 3-month period were consecutively approached to participate in a semistructured interview on the care-seeking process. Interviews were conducted in Swahili by a native speaker. A total of 58 caregivers were eligible, with a 100% participation rate. Seven interviews were incomplete and excluded from subsequent analysis. In one interview, it could not be ascertained when the child first received treatment at BMC, and so the interview was excluded. One interview had a referral delay > 3 standard deviations over the mean travel time and was also excluded. In total, data from 49 patients were reviewed. Patient age, sex, and diagnosis were available for 47 patients (96%).
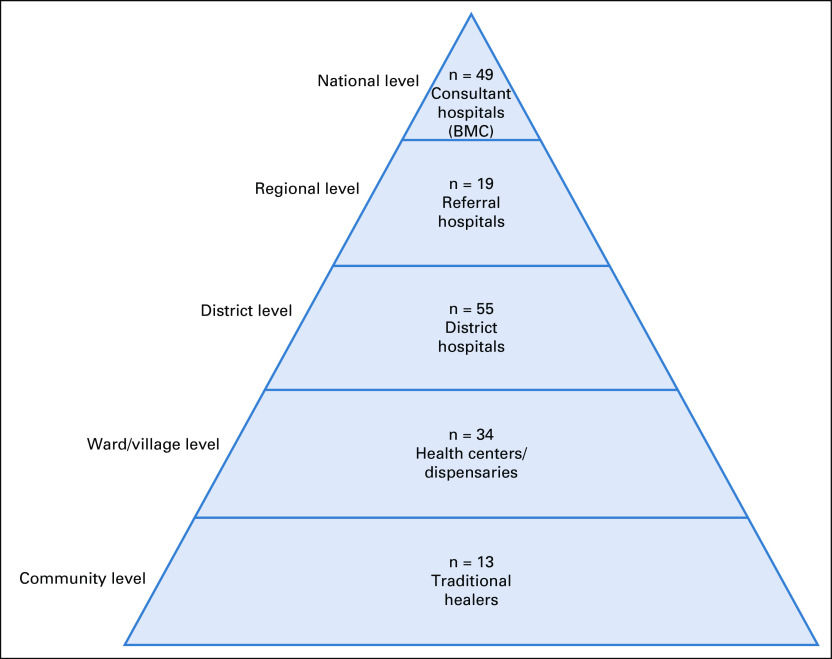
Caregivers were asked to identify each health facility visited before reaching BMC. Facility types were grouped into levels on the basis of guidelines from the Tanzanian Ministry of Health (Fig 1), including traditional healer, duka la dawa (pharmacy), dispensary, health center, district hospital, private hospital, referral hospital, and consultant hospital. Dispensaries serve as the first point of health care for villages. From there, patients are supposed to be referred to health centers, then district hospitals, then referral hospitals, and finally consultant hospitals.30
FIG 1.
The tiered Tanzanian health care system based on guidelines set by the Tanzanian Ministry of Health, Community Development, Gender, Elderly and Children.30 Facility types that patients reported in this study are grouped into their respective tier. The number of facility visits to each facility type is also shown. Of note, the only consultant hospital visited by patients was the Bugando Medical Centre (BMC).
Because caregivers are referred to dispensaries and health centers interchangeably, the two categories were grouped together for analysis. In addition, the visits to private pharmacies (n = 2) and private hospitals (n = 1) were excluded from analysis of the impact of facility type on outcomes because the total number of visits to these facility types was too low to provide accurate statistical representation.
Caregivers were asked to provide the estimated date of attendance to each facility and whether a referral was made during that visit. If only a month could be provided, the first day of the month was assumed for statistical analysis. Services received at each health facility visited (laboratory tests, imaging, etc) and means of transportation used to reach the facility were recorded as well.
Definition of Referral Delay
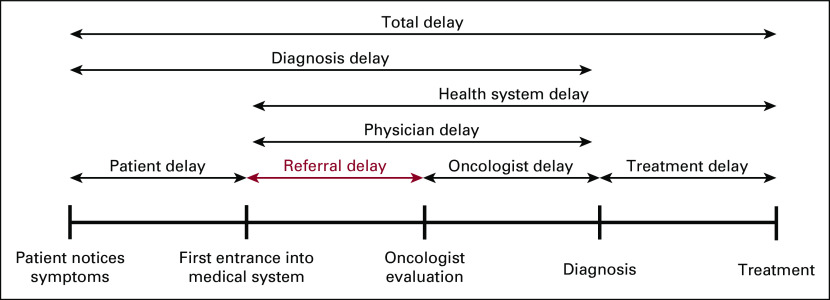
To aid in understanding the causal factors of late presentation, a standardized published framework was used to break down the period from patients noticing cancer symptoms until treatment initiation into separate types of delays.31 While there are various types of delay (Fig 2), this study centered on referral delay, defined as the number of days between when a patient first visited any type of health facility to the time the patient was seen at the oncology department of BMC.
FIG 2.
Visual representation of various types of delays in the process of seeking and undergoing cancer care. Referral delay is highlighted in red. Adapted from a framework developed by Dang-Tan et al.31
Statistical Analysis
Descriptive statistical analysis was performed to determine the average and median referral delay time as well as the average and median number of facilities visited for all patients. The impact of the first health care facility visited was examined by comparing time and number of facilities remaining until reaching BMC on the basis of first type of facility visited. Analysis of services received and transportation used to reach each facility was also compared between types of facilities visited. The impact of facility referral on time to presentation was evaluated by type (ie, none received, referred to higher-level facility). Statistical analyses were performed with RStudio software (RStudio, Boston, MA). All P values were calculated using the Mann-Whitney U test and considered significant at P < .05.
Ethical Considerations
The study was approved by the Catholic University of Health and Allied Sciences/BMC Research Ethical Committee (Mwanza, Tanzania) as well as by the National Institute for Medical Research-Lake Zone Medical Research Coordinating Committee.
RESULTS
The median patient age was 5 years, ranging from 10 months to 18 years, and 51% of patients were female. Solid tumors comprised the majority of diagnoses (63%, n = 27) followed by hematologic malignancies (35%, n = 15), and CNS malignancy (2%, n = 1). Diagnosis was unknown in four patients. This diagnosis distribution was representative of the actual distribution of solid and hematologic malignancies seen at BMC (70% and 30%, respectively).
The median referral delay was 89 days (mean, 121 days). Referral delay time did not vary significantly between males and females (133 v 96 days; P = .38) or solid and hematologic tumors (141 v 90 days; P = .32). The median number of facilities visited before reaching BMC was two (mean, 2.5 facilities). Of 124 total facility visits, the most commonly visited facility type was district hospital (44%, n = 55) followed by health center/dispensary (27%, n = 34), referral hospital (15%, n = 19), traditional healer (10%, n = 13), duka la dawa (2%, n = 2), and private hospital (1%, n = 1).
The most common point of entry to the health care system was health center/dispensary (41%, n = 20) followed by district hospital (35%, n = 17), traditional healer (10%, n = 5), and referral hospital (6%, n = 3). The total time to BMC was significantly longer for patients who started at a traditional healer than for patients who started at a district hospital (P = .046) or health center/dispensary (P = .02; Table 1). There was no significant difference in time to BMC among any other facility types. There was also no significant difference in total number of facilities visited before reaching BMC for patients, regardless of what facility type a patient started at.
TABLE 1.
Impact of Point-of-Entry Facility on Time to BMC and Facilities to BMC

The type of facility a patient went to after a facility visit is shown in Figure 3. In 95% of visits to a referral hospital, the next facility visited was BMC compared with 36% for a district hospital visit, 24% for a health center/dispensary visit, and 8% for a traditional healer visit.
FIG 3.
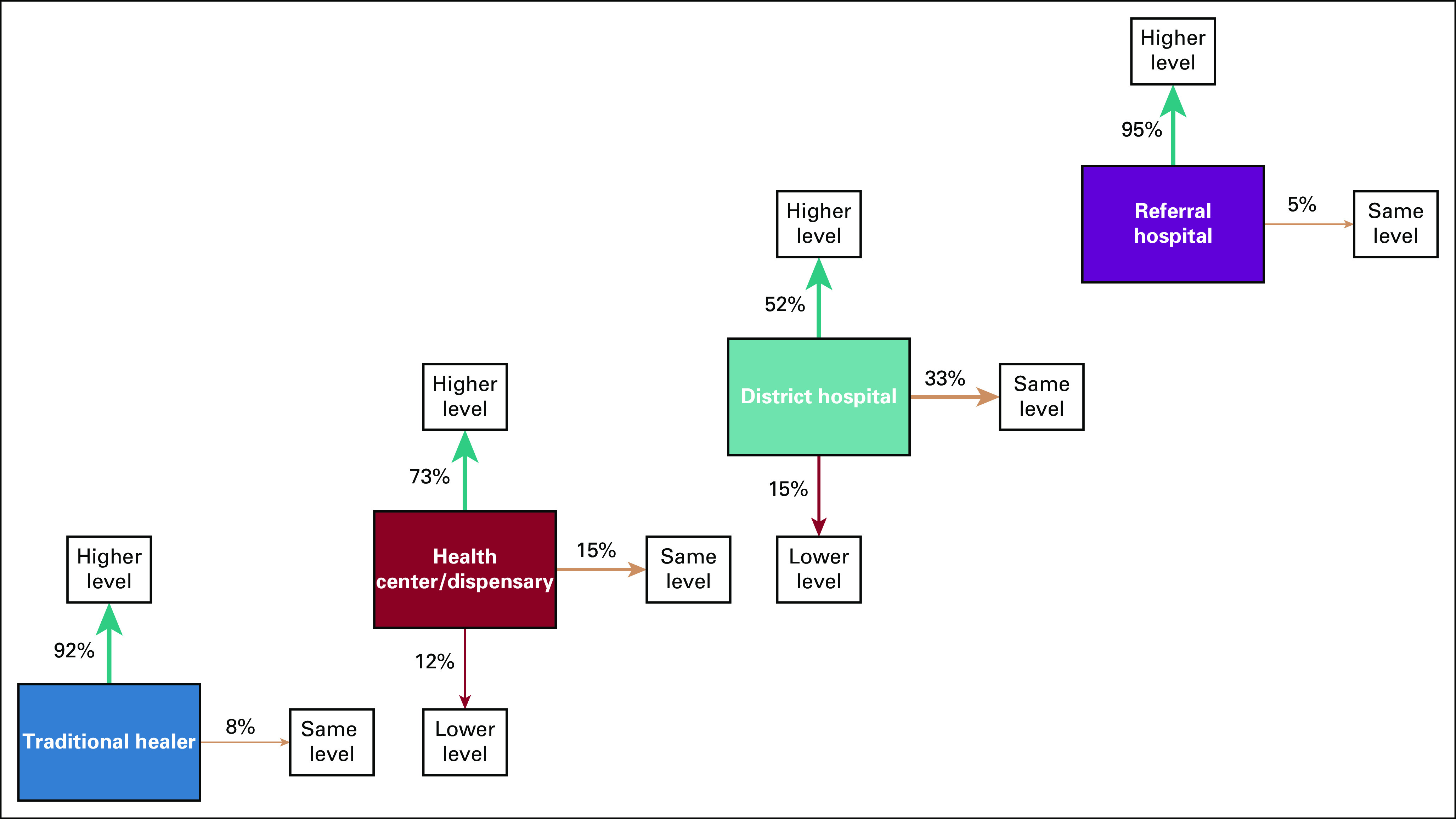
Effect of type of facility visited on level of next facility visited. Numbers were normalized by total visits to each facility type.
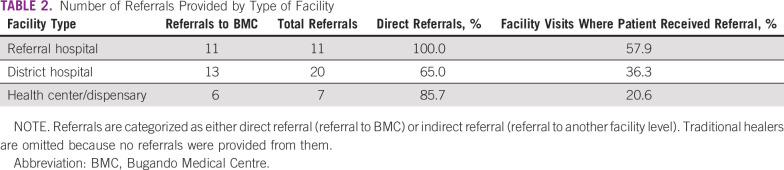
In total, 65% of patients (n = 32) received a referral at some point in their care-seeking process. The remaining 34% (n = 17) self-referred to each facility they visited. Of the 38 total referrals, 53% (n = 20) were from district hospitals, 29% (n = 11) from referral hospitals, and 18% (n = 7) from health centers/dispensaries. Of 38 total referrals, 79% (n = 30) were directly to BMC and 21% (n = 8) to other facilities. Eighty-nine percent of referrals (n = 34) were to a higher-level facility, and the remaining 11% (n = 4) were from one district hospital to another. Table 2 lists an overview of the type of referrals given by facility level and the frequency of referral based on facility type.
TABLE 2.
Number of Referrals Provided by Type of Facility
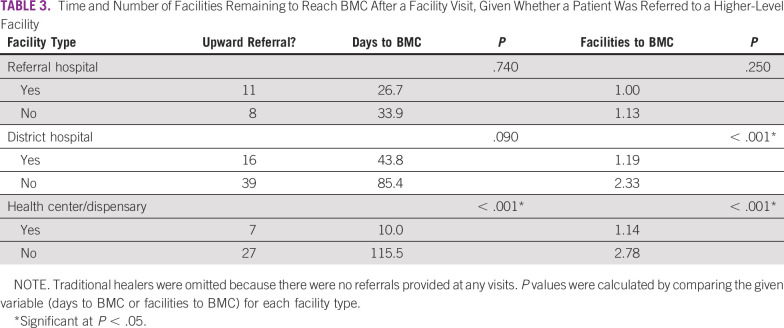
The average time left to BMC after a facility visit was significantly lower for visits where a patient received a referral to a higher-level facility than visits where they did not (31.3 v 99.3 days; P < .001). The same was true of the number of facilities remaining until reaching BMC (1.12 v 2.44; P < .001). Table 3 lists a comparison of the time and number of facilities remaining until reaching BMC by facility type and whether the facility visit resulted in an upward referral.
TABLE 3.
Time and Number of Facilities Remaining to Reach BMC After a Facility Visit, Given Whether a Patient Was Referred to a Higher-Level Facility
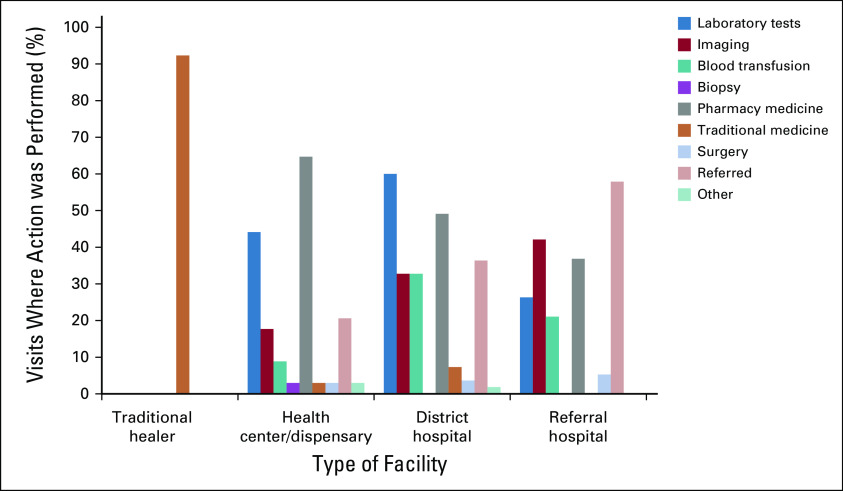
Data on interventions received based on type of facility are shown in Figure 4. Overall, the most common interventions received were pharmacy medication (47%, n = 58) and laboratory investigations (43%, n = 53). Biopsy was the least common intervention (1%, n = 1). Most patients received at least one intervention (96%, n = 47) before presentation at BMC. The average number of interventions per facility was 1.86, ranging from 0.9 for traditional healer to 2.2 for district hospitals.
FIG 4.
Actions taken at each type of facility. The nine-category system for classifying interventions that patients received is presented in the figure key. Results are normalized by number of visits to type of facility.
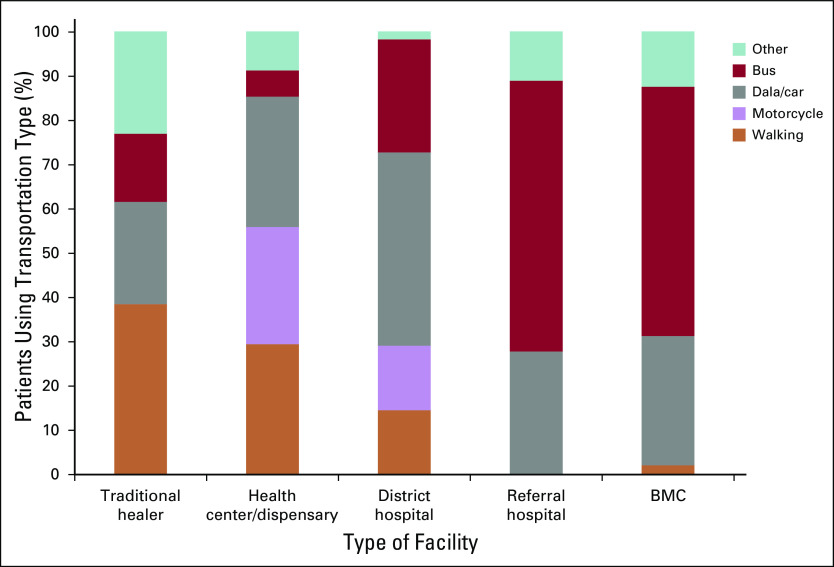
The type of transportation used to reach each health facility is shown in Figure 5. Motorized transportation (motorcycle, dala/car, or bus) was the main form of transportation used to visit every type of facility except traditional healers. For traditional healers, a majority of patients (62%) walked, biked, or were visited at home.
FIG 5.
Transportation used to reach each facility type. Other transportation for traditional healer visits and health center/dispensary visits means treated at home or traveled by bicycle, whereas it means traveled by ambulance for district hospital, referral hospital, or Bugando Medical Centre (BMC) visits.
DISCUSSION
The late presentation of patients with pediatric cancer to a cancer treatment facility is common in SSA.16 Late presentation, however, is due to a combination of patient delay (delay in deciding to seek care) and referral delay (delays while navigating the health system). This delay allows for disease progression and ultimately can worsen treatment outcomes.17,18 In the current study, we found that delays within the hospital system were extensive, resulting in a median referral delay of 89 days. Two patients had referral delays > 1 year. To our knowledge, this study is the first to examine the referral delay component of late presentation for patients with pediatric cancer in SSA.
To reduce health system inefficiencies and improve referral delay, it is important to target the point of entry within the medical system. More than 75% of patients in our region began the care-seeking process at health centers/dispensaries or district hospitals. Because so many patients start at these facilities, it is concerning that 48% of district hospital visits and 27% of health centers/dispensary visits were followed by a visit to a same-level or lower-level facility.
This lack of upward facility movement for patients with pediatric cancer who visit these facilities contributes to referral delay because there is no additional testing or diagnostic interventions when moving to a same-level or lower-level facility. In Tanzania, the referral process is designed so that a patient with a disease that requires specialized treatment who enters the health system at a lower facility level should be referred to a higher facility level. For example, if a child visits a health center and is suspected to have cancer, the child should be sent to a district or referral hospital for further evaluation and diagnostic testing. The results of this study show that in practice, this is often not happening for patients with pediatric cancer.
Also concerning is that only 36% of visits to a district hospital and 21% of visits to a health center/dispensary resulted in a patient getting referred. When a referral to a higher-level facility was given to a patient, however, the average time to BMC and facilities left to visit before BMC were significantly lower than when no upward referral was given. Taken together with the results described for where patients go after a health facility visit, these findings suggest that educating health care workers at health centers/dispensaries and district hospitals to recognize cancer and refer patients upward could help to reduce referral delay.
There are several health system interventions in Tanzania and SSA more broadly that could serve as examples of how to do this. The training of lay personnel to recognize early signs of cancer in Tanzania and refer patients to a health care provider if they find a suspected malignancy has resulted in patients presenting at significantly earlier stages.32 A cancer education program targeted to primary health care workers in Botswana has been shown to significantly increase participant knowledge.33 Rwanda’s decentralized system for referring injured patients for surgery completed > 50% of referrals from rural district hospitals in a timely manner.34 Furthermore, the Academic Model Providing Access to Healthcare, which once focused exclusively on HIV, has leveraged its referral system in Kenya to refer patients with cancer, which has led to a dramatic increase in the number of referred patients and serves as a case study on how to strengthen health systems for patients with cancer.35
Although a less frequent starting facility, patients who started at traditional healers were found to have significantly longer delays than those who entered at a district hospital or health center/dispensary. This is consistent with other studies in SSA that found that seeking care from a traditional healer is a cause of late presentation. For example, visiting traditional healers has led to delays in cancer treatment in Ghana,36 late diagnosis in Ethiopia,37 and longer referral delay in Rwanda.38 This is probably due to the lack of diagnostics available at traditional healers. In our cohort, no patients received a diagnostic intervention (laboratory tests, imaging, or biopsy) at a healer. Instead, only traditional medicine was provided.
Results on transportation, however, highlight that traditional healers are highly accessible. In most cases (62%), patients were able to walk, ride a bike, or be visited at home instead of having to spend money on motorized transportation. For all other facility levels, the increasing proportion of patients who used motorized transportation to reach a facility for higher facility levels points to transportation as a potential barrier for families seeking cancer care, as has been previously recognized.39
Traditional healers have long been recognized as an integral part of the health care in SSA.40,41 The accessibility and importance of healers in communities suggest that healers should also be incorporated into training efforts for cancer recognition and efforts to better connect health care workers with BMC. The inclusion of healers in these efforts could also help to decrease referral delay. Prior collaborative work between government health systems and traditional healers to combat the HIV epidemic could serve as a model for such efforts.40,41
Along with time to reach BMC, cost may be an important component of the barriers patients face in reaching BMC. Eighty-two percent of patients received treatment (pharmacy or traditional) before presentation at BMC. Because cancer treatment is not available at any facilities except BMC, the treatments were only for symptom management and could be a source of increased health care cost burden on families. The failure of a facility to provide a correct diagnosis, as evidenced by the low number of facilities that referred patients to the cancer treatment center, also increases the number of facility visits and total transportation costs.
In Tanzania, where 49% of the population lives below US $1.90 a day, the compounding of these direct costs of transportation, incorrect medications, and multiple facility visits with the indirect costs of lost wages from caregivers’ time away from work can be catastrophic.42 Financial hardship is known to contribute significantly to abandonment of care for patients with pediatric cancer.43 Efforts to strengthen connections between lower-level health facilities and BMC could help to reduce both direct and indirect costs.
While it is not possible to interview patients who did not reach care, BMC only sees an estimated 15% of patients with predicted pediatric cancer in their coverage area, despite being the only cancer treatment facility.44 Referral delay along with associated direct and indirect costs of care before reaching BMC may contribute to the patients abandoning care seeking. Additional study is needed to better understand why BMC sees so few of the patients with predicted pediatric cancer in its coverage area.
This study was limited in several ways. First, guardians were not asked about their income, education, or employment. Future studies into whether these factors may influence referral delay are needed. Furthermore, guardians were not asked whether they owned a transportation vehicle. A survey that further examines modes of transportation used by patients may be beneficial in understanding what factors influence referral delay.
Overall, the majority of patients with pediatric cancer in northern Tanzania experience delays during the referral process. To downstage patients and reduce the costs associated with seeking care, systems will need to be strengthened to better connect all facility levels to the cancer referral center and ensure that all patients are referred to care in a timely manner.
ACKNOWLEDGMENT
We thank the BMC Oncology Department and the Bugando Duke collaboration for supporting this project. We also thank Franco Afyusisye for help with accessing the patient registry. Finally, we thank Katie Barrett for her assistance in editing this article.
SUPPORT
Supported by the US Fulbright Program (L.M.).
AUTHOR CONTRIBUTIONS
Conception and design: Luke Maillie, Nestory Masalu, Kristin Schroeder
Administrative support: Nestory Masalu
Provision of study material or patients: Nestory Masalu, Kristin Schroeder
Collection and assembly of data: All authors
Data analysis and interpretation: Luke Maillie, Nestory Masalu, Kristin Schroeder
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/site/misc/authors.html.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
No potential conflicts of interest were reported.
REFERENCES
- 1.Ward ZJ, Yeh JM, Bhakta N, et al. Estimating the total incidence of global childhood cancer: A simulation-based analysis. Lancet Oncol. 2019;20:483–493. doi: 10.1016/S1470-2045(18)30909-4. [DOI] [PubMed] [Google Scholar]
- 2. Howlader N, Noone AM, Krapcho M, et al (eds): SEER Cancer Statistics Review, 1975-2017, Bethesda, MD, National Cancer Institute, 2019. Available from https://seer.cancer.gov/csr/1975_2017.
- 3.Smith MA, Seibel NL, Altekruse SF, et al. Outcomes for children and adolescents with cancer: Challenges for the twenty-first century. J Clin Oncol. 2010;28:2625–2634. doi: 10.1200/JCO.2009.27.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kellie SJ, Howard SC. Global child health priorities: What role for paediatric oncologists? Eur J Cancer. 2008;44:2388–2396. doi: 10.1016/j.ejca.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 5.Ribeiro RC, Steliarova-Foucher E, Magrath I, et al. Baseline status of paediatric oncology care in ten low-income or mid-income countries receiving My Child Matters support: A descriptive study. Lancet Oncol. 2008;9:721–729. doi: 10.1016/S1470-2045(08)70194-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hadley LG, Rouma BS, Saad-Eldin Y. Challenge of pediatric oncology in Africa. Semin Pediatr Surg. 2012;21:136–141. doi: 10.1053/j.sempedsurg.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Farmer P, Frenk J, Knaul FM, et al. Expansion of cancer care and control in countries of low and middle income: A call to action. Lancet. 2010;376:1186–1193. doi: 10.1016/S0140-6736(10)61152-X. [DOI] [PubMed] [Google Scholar]
- 8.Israëls T, Chirambo C, Caron HN, et al. Nutritional status at admission of children with cancer in Malawi. Pediatr Blood Cancer. 2008;51:626–628. doi: 10.1002/pbc.21697. [DOI] [PubMed] [Google Scholar]
- 9.Friedrich P, Lam CG, Itriago E, et al. Magnitude of treatment abandonment in childhood cancer. PLoS One. 2015;10:e0135230. doi: 10.1371/journal.pone.0135230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stefan DC. Cancer care in Africa: An overview of resources. J Glob Oncol. 2015;1:30–36. doi: 10.1200/JGO.2015.000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schonland M, Bradshaw E. Oesophageal cancer in Natal Bantu: A review of 516 cases. S Afr Med J. 1969;43:1028–1031. [PubMed] [Google Scholar]
- 12.Gelfand M, Graham AJ, Lightman E. Carcinoma of bronchus and the smoking habit in Rhodesian Africans. BMJ. 1968;3:468–469. doi: 10.1136/bmj.3.5616.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vogel CL, Bayley AC, Brooker RJ, et al. A phase II study of Adriamycin (NSC 123127) in patients with hepatocellular carcinoma from Zambia and the United States. Cancer. 1977;39:1923–1929. doi: 10.1002/1097-0142(197705)39:5<1923::aid-cncr2820390502>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 14.Olweny CL, Katongole-Mbidde E, Bahendeka S, et al. Further experience in treating patients with hepatocellular carcinoma in Uganda. Cancer. 1980;46:2717–2722. doi: 10.1002/1097-0142(19801215)46:12<2717::aid-cncr2820461230>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 15.Sobo AO. Cancer in Liberia: A review of cases registered from the Liberia Cancer Registry 1973-1977. Cancer. 1982;49:1945–1951. doi: 10.1002/1097-0142(19820501)49:9<1945::aid-cncr2820490933>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 16.Stefan C, Bray F, Ferlay J, et al. Cancer of childhood in sub-Saharan Africa. Ecancermedicalscience. 2017;11:755. doi: 10.3332/ecancer.2017.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chantada GL, Qaddoumi I, Canturk S, et al. Strategies to manage retinoblastoma in developing countries. Pediatr Blood Cancer. 2011;56:341–348. doi: 10.1002/pbc.22843. [DOI] [PubMed] [Google Scholar]
- 18.Orem J, Mulumba Y, Algeri S, et al. Clinical characteristics, treatment and outcome of childhood Burkitt’s lymphoma at the Uganda Cancer Institute. Trans R Soc Trop Med Hyg. 2011;105:717–726. doi: 10.1016/j.trstmh.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Magrath I, Steliarova-Foucher E, Epelman S, et al. Paediatric cancer in low-income and middle-income countries. Lancet Oncol. 2013;14:e104–e116. doi: 10.1016/S1470-2045(13)70008-1. [DOI] [PubMed] [Google Scholar]
- 20. Gelband H, Jha P, Sankaranarayanan R, et al (eds): Cancer: Disease Control Priorities (ed 3). Vol 3. Washington, DC, The International Bank for Reconstruction and Development/The World Bank, 2015. [PubMed] [Google Scholar]
- 21. Saxton AT, Bhattacharya M, Masalu N, et al: Cost-effectiveness of pediatric cancer treatment in Tanzania: An economic analysis. J Glob Oncol 10.1200/JGO.2017.009480. [Google Scholar]
- 22.Traoré F, Coze C, Atteby JJ, et al. Cyclophosphamide monotherapy in children with Burkitt lymphoma: A study from the French-African Pediatric Oncology Group (GFAOP) Pediatr Blood Cancer. 2011;56:70–76. doi: 10.1002/pbc.22746. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez-Galindo C, Friedrich P, Morrissey L, et al. Global challenges in pediatric oncology. Curr Opin Pediatr. 2013;25:3–15. doi: 10.1097/MOP.0b013e32835c1cbe. [DOI] [PubMed] [Google Scholar]
- 24.Brown CA, Kohler RE, John O, et al. Multilevel Factors affecting time to cancer diagnosis and care quality in Botswana. Oncologist. 2018;23:1453–1460. doi: 10.1634/theoncologist.2017-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Suneja G, Dryden-Peterson S, Boyer M, et al: Cancer in Botswana: A prospective cohort study of cancer type, treatment, and outcomes. Int J Rad Oncol Biol Phys 87:S492-S493, 2013. [Google Scholar]
- 26.Gilyoma JM, Rambau PF, Masalu N, et al. Head and neck cancers: A clinico-pathological profile and management challenges in a resource-limited setting. BMC Res Notes. 2015;8:772. doi: 10.1186/s13104-015-1773-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chalya PL, Rambau PF, Masalu N, et al. Ten-year surgical experiences with penile cancer at a tertiary care hospital in northwestern Tanzania: A retrospective study of 236 patients. World J Surg Oncol. 2015;13:71. doi: 10.1186/s12957-015-0482-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mabula JB, McHembe MD, Koy M, et al. Gastric cancer at a university teaching hospital in northwestern Tanzania: A retrospective review of 232 cases. World J Surg Oncol. 2012;10:257. doi: 10.1186/1477-7819-10-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chalya PL, McHembe MD, Mabula JB, et al. Clinicopathological patterns and challenges of management of colorectal cancer in a resource-limited setting: A Tanzanian experience. World J Surg Oncol. 2013;11:88. doi: 10.1186/1477-7819-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ministry of Health, Community Development, Gender, Elderly and Children: The National Health Policy 2017. Dar es Salaam, Tanzania, The United Republic of Tanzania, 2017. [Google Scholar]
- 31.Dang-Tan T, Trottier H, Mery LS, et al. Delays in diagnosis and treatment among children and adolescents with cancer in Canada. Pediatr Blood Cancer. 2008;51:468–474. doi: 10.1002/pbc.21600. [DOI] [PubMed] [Google Scholar]
- 32.Ngoma T, Mandeli J, Holland JF. Downstaging cancer in rural Africa. Int J Cancer. 2015;136:2875–2879. doi: 10.1002/ijc.29348. [DOI] [PubMed] [Google Scholar]
- 33.Tapela NM, Peluso MJ, Kohler RE, et al. A step toward timely referral and early diagnosis of cancer: Implementation and impact on knowledge of a primary care-based training program in Botswana. Front Oncol. 2018;8:187. doi: 10.3389/fonc.2018.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nkurunziza T, Toma G, Odhiambo J, et al. Referral patterns and predictors of referral delays for patients with traumatic injuries in rural Rwanda. Surgery. 2016;160:1636–1644. doi: 10.1016/j.surg.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 35.Strother RM, Asirwa FC, Busakhala NB, et al. AMPATH-Oncology: A model for comprehensive cancer care in sub-Saharan Africa. J Cancer Policy. 2013;1:e42–e48. [Google Scholar]
- 36.Clegg-Lamptey JN, Dakubo JC, Attobra YN. Psychosocial aspects of breast cancer treatment in Accra, Ghana. East Afr Med J. 2009;86:348–353. doi: 10.4314/eamj.v86i7.54152. [DOI] [PubMed] [Google Scholar]
- 37.Gebremariam A, Addissie A, Worku A, et al. Perspectives of patients, family members, and health care providers on late diagnosis of breast cancer in Ethiopia: A qualitative study. PLoS One. 2019;14:e0220769. doi: 10.1371/journal.pone.0220769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pace LE, Mpunga T, Hategekimana V, et al. Delays in breast cancer presentation and diagnosis at two rural cancer referral centers in Rwanda. Oncologist. 2015;20:780–788. doi: 10.1634/theoncologist.2014-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harford JB. Barriers to overcome for effective cancer control in Africa. Lancet Oncol. 2015;16:e385–e393. doi: 10.1016/S1470-2045(15)00160-6. [DOI] [PubMed] [Google Scholar]
- 40.Mills E, Singh S, Wilson K, et al. The challenges of involving traditional healers in HIV/AIDS care. Int J STD AIDS. 2006;17:360–363. doi: 10.1258/095646206777323382. [DOI] [PubMed] [Google Scholar]
- 41. King R, Homsy J: Involving traditional healers in AIDS education and counselling in sub-Saharan Africa: A review. Aids 11:S217-S225, 1997. [PubMed] [Google Scholar]
- 42. The World Bank: Poverty & Equity Data Portal, 2019. http://povertydata.worldbank.org.
- 43.Gupta S, Yeh S, Martiniuk A, et al. The magnitude and predictors of abandonment of therapy in paediatric acute leukaemia in middle-income countries: A systematic review and meta-analysis. Eur J Cancer. 2013;49:2555–2564. doi: 10.1016/j.ejca.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 44. doi: 10.1200/JGO.2016.009027. Schroeder K, Saxton A, McDade J, et al: Pediatric cancer in northern Tanzania: Evaluation of diagnosis, treatment, and outcomes. J Glob Oncol 10.1200/JGO.2016.009027. [DOI] [PMC free article] [PubMed] [Google Scholar]