Figure 2. Baseline amyloid status changes the effect of escitalopram on reduction in CSF β-amyloid (Aβ)42.
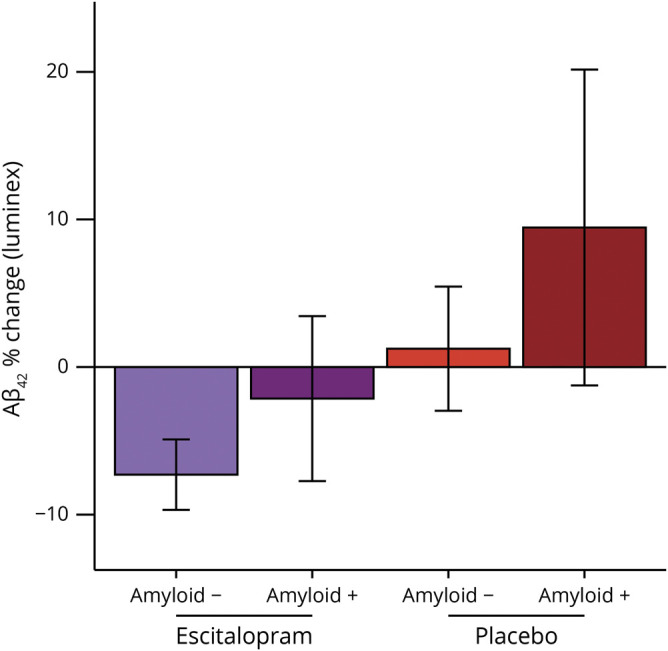
Participants who were amyloid positive at baseline (pooled) had significantly less percent reduction in Aβ42 (F1,107 = 7.968, p = 0.006, 95% confidence interval −16.7% to −0.5%, d = −0.52), controlling for age, sex, and treatment group (not pictured). On average (pictured), amyloid-negative escitalopram-treated (combined doses) patients had a 7.3% (SEM 1.2%) decrease; amyloid-positive escitalopram-treated patients had a 2.1% (SEM 2.9%) decrease; amyloid-negative placebo-treated patients had a 1.2% (SEM 2.2%) increase; amyloid-positive placebo-treated patients had a 9.5% (SEM 5.5%) increase.
