Abstract
Neuromyelitis optica spectrum disorders are a group of rare, but severe autoimmune diseases characterized by inflammation of the optic nerve(s) and/or spinal cord. Although naive B cells are considered key players by escaping central tolerance checkpoints, it remains unclear how their composition and outgrowth differ in patients with neuromyelitis optica spectrum disorders. Under complete treatment-naive circumstances, we found that naive mature/transitional B-cell ratios were reduced in the blood of 10 patients with aquaporin-4 immunoglobulin G-positive disease (neuromyelitis optica spectrum disorders) as compared to 11 both age- and gender-matched healthy controls, eight patients with myelin oligodendrocyte glycoprotein-immunoglobulin G-associated disorders and 10 patients with multiple sclerosis. This was the result of increased proportions of transitional B cells, which were the highest in patients with neuromyelitis optica spectrum disorders with relapses and strongly diminished in a separate group of nine patients with neuromyelitis optica spectrum disorders and myelin oligodendrocyte glycoprotein-immunoglobulin G-associated disorders who received corticosteroid treatment. These findings need to be confirmed in longitudinal studies. For purified naive mature B cells of seven patients with neuromyelitis optica spectrum disorders and myelin oligodendrocyte glycoprotein-immunoglobulin G-associated disorders with relapses, Toll-like receptor 9 ligand synergized with interferon-γ to enhance plasmablast formation during germinal centre-like cultures. This was not seen for 11 patients without relapses and nine healthy controls. In the neuromyelitis optica spectrum disorders group, in vitro plasmablast formation corresponded to total and anti-aquaporin-4 immunoglobulin G secretion, of which the latter was found only for relapsing cases. These data indicate that naive B-cell homoeostasis is different and selectively targeted by corticosteroids in patients with neuromyelitis optica spectrum disorders. This also supports further exploration of naive B cells for their use in Toll-like receptor 9-dependent in vitro platforms in order to predict the activity of neuromyelitis optica spectrum disorders.
Keywords: aquaporin-4, myelin oligodendrocyte glycoprotein, plasmablasts, TLR9, transitional B cells
Circulating transitional and not naive mature B cells show increased frequencies and seem to be selectively targeted by corticosteroids in patients with aquaporin-4 immunoglobulin G-positive neuromyelitis optica spectrum disorders. Naive mature B cells of patients with relapses preferentially develop into aquaporin-4 immunoglobulin G-producing plasmablasts in vitro under interferon-gamma- and Toll-like receptor 9-inducing conditions.
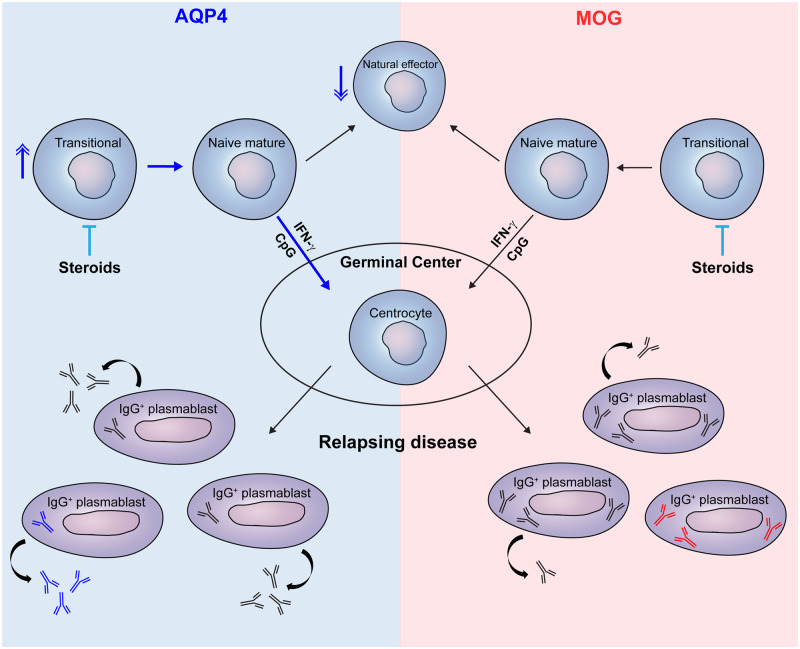
Graphical Abstract
Graphical Abstract.
Introduction
Neuromyelitis optica spectrum disorders (NMOSD) are rare and convey a range of severe clinical presentations caused by inflammation with preferential involvement of the optic nerves and spinal cord (Wingerchuk et al., 2007). Although the exact cause of these divergent presentations remains poorly understood, the dominant role of the B-cell lineage is undisputed (Sabatino et al., 2019). In approximately 75% of NMOSD patients, immunoglobulin G (IgG) antibodies are found that target the neuronal water channel protein aquaporin-4 (AQP4; Saadoun and Papadopoulos, 2010). Furthermore, 30–40% of AQP4-IgG-negative NMOSD patients test positive for antibodies against myelin oligodendrocyte glycoprotein (MOG; Pelt van et al., 2016a; Hamid et al., 2017), which are associated with a distinct entity termed MOG-IgG-associated disorders (MOGAD).
There are significant differences in clinical features between AQP4-IgG-positive NMOSD and MOGAD (Kitley et al., 2012; Pelt van et al., 2016b; Jurynczyk et al., 2017), including a higher frequency and worse recovery from relapses in AQP4-IgG-positive NMOSD. Relapses are commonly treated with corticosteroids in both entities. To prevent relapses, AQP4-IgG-positive NMOSD patients and relapsing patients with MOGAD are usually treated with maintenance therapy. Currently, no biomarkers are available to accurately predict relapses and guide treatment decisions. This could be due to the fact that previous studies on the immunopathogenesis of NMOSD primarily used patients treated with corticosteroids or other maintenance therapy, and hence possibly obliterating disease-relevant B-cell subsets.
Recent findings reveal that AQP4-specific B cells are already present in naive populations that escape early tolerance checkpoints (Wilson et al., 2018; Cotzomi et al., 2019). Normally, self-reactive clones are counterselected during early B-cell development in the bone marrow (central tolerance) and during subsequent maturation of transitional into naive mature B cells after entering the circulation (peripheral tolerance). In patients with AQP4-IgG-positive NMOSD, naive mature B cells escape both these checkpoints and likely develop into antibody-secreting cells in a germinal centre-dependent manner (Kowarik et al., 2017; Wilson et al., 2018; Cotzomi et al., 2019). In systemic autoimmune disease, which coexists in ∼20% of AQP4-IgG-positive NMOSD patients (Shahmohammadi et al., 2019), interleukin (IL)-21, interferon (IFN)-γ and Toll-like receptor (TLR9)-ligand CpG-ODN serve as key triggers of auto-reactive germinal centre B cells expressing T-box transcription factor T-bet (Sindhava et al., 2017; Wang et al., 2018).
In this study, we aimed to define the impact of AQP4-IgG serostatus, steroid treatment and relapse occurrence on naive B-cell development in NMOSD. The composition of the naive B-cell pool within the blood was compared between NMOSD, MOGAD and multiple sclerosis groups with and without corticosteroid treatment, as well as matched healthy controls. Furthermore, naive B-cell outgrowth into (anti-AQP4 or -MOG) IgG-secreting plasmablasts was explored in vitro for patients with and without relapses during T-bet-inducing, germinal centre-like cultures.
Materials and methods
Participants
We included 10 treatment-naive AQP4-IgG-positive NMOSD patients (Wingerchuk et al., 2015) and eight treatment-naive patients with MOGAD (all with optic neuritis and/or transverse myelitis). These patients did not get immune suppressive therapy before blood sampling; no steroids within 1 month and no other maintenance treatment within 3 months. Additionally, five patients with AQP4-IgG-positive NMOSD and four patients with MOGAD were included who received corticosteroids (i.e. oral prednisone or intravenous methylprednisolone) within 1 month prior to sampling. In the corticosteroid-treated MOGAD group, two patients had an NMOSD phenotype, one patient presented with acute disseminated encephalomyelitis and one patient was diagnosed with encephalitis. None of the patients included in this study received therapy with prolonged immune suppressive activity, such as anti-CD20 or any other B-cell-directed monoclonal antibodies before sampling. Patient characteristics are summarized in Table 1.
Table 1.
Patient characteristics
| Cohort | Agea (years)/ Gender | Disease location onset + location relapse | Treatmenta,b | Time since first eventa (months) | Relapse | Serum AQP4-IgG/ MOG-IgG level (ΔMFI)a,c | Ex vivo subgroup | Used in vitro |
|---|---|---|---|---|---|---|---|---|
| AQP4-IgG | ||||||||
| Patient 1 | 41/F | ON + ON | None | 7 | Yes | 7644 | NMOSD | Yes |
| Patient 2 | 39/F | TM + 2×TM | None | 78 | Yes | 13 365 | NMOSD | Yes |
| Patient 3 | 56/F | ON bilateral + ON/BS | None | 6 | Yes | 13 736 | NMOSD | Yes |
| Patient 4 | 61/F | TM | None | 5 | No | 7469 | NMOSD | Yes |
| Patient 5 | 46/M | ON bilateral | None | 15 | No | 545 | NMOSD | Yes |
| Patient 6 | 47/F | ON | None | 2 | No | 15 437 | NMOSD | Yes |
| Patient 7 | 27/F | TM | None | 46 | No | 7874 | NMOSD | Yes |
| Patient 8 | 26/F | BS | None | 122 | No | 7262 | NMOSD | Yes |
| Patient 9 | 36/F | ON | None | 0 | No | ND | NMOSD | Yes |
| Patient 10 | 46/F | TM | None | 4 | No | 20 533 | NMOSD | No |
| Patient 11 | 34/F | TM + TM | P | 5 | Yes | 82 | CS-treated | No |
| Patient 12 | 50/F | TM + 2×TM | MP | 13 | Yes | 17 061 | CS-treated | No |
| Patient 13 | 19/F | BS + TM | MP + P | 6 | Yes | 14 055 | CS-treated | No |
| Patient 14 | 58/F | TM/ON bilateral | MP + P | 2 | No | 15 618 | CS-treated | No |
| Patient 15 | 37/F | BS/ON bilateral | MP + P | 4 | No | 16 701 | CS-treated | No |
| MOG-IgG | ||||||||
| Patient 1 | 38/F | ON + 5×ON | None | 95 | Yes | 24 237 | MOGAD | Yes |
| Patient 2 | 26/M | ON + ON | None | 25 | Yes | 5099 | MOGAD | Yes |
| Patient 3 | 25/F | TM + 2×ON | None | 17 | Yes | 424 | MOGAD | Yes |
| Patient 4 | 32/M | ADEM + TM | MP | 326 | Yes | 1681 | CS-treated | Yes |
| Patient 5 | 52/M | ON | None | 10 | No | 136 | MOGAD | Yes |
| Patient 6 | 40/F | ON bilateral | None | 10 | No | 1234 | MOGAD | Yes |
| Patient 7 | 56/F | TM | None | 18 | No | 696 | MOGAD | Yes |
| Patient 8 | 37/M | TM/ON | MP | 1 | No | 6495 | CS-treated | Yes |
| Patient 9 | 26/F | ON bilateral | MP + P | 1 | No | 2058 | CS-treated | Yes |
| Patient 10 | 25/F | ON bilateral + 3×ON | None | 201 | Yes | 6617 | MOGAD | No |
| Patient 11 | 32/M | E + E | MP | 181 | Yes | 21 | CS-treated | No |
| Patient 12 | 29/M | TM | None | 51 | No | 1200 | MOGAD | No |
ADEM, acute disseminated encephalomyelitis; BS, brainstem (area postrema or cranial nerves); CS, corticosteroid; E, encephalitis; MFI, mean fluorescence intensity; MP, methylprednisolone (intravenous); ND, not determined; ON, optic neuritis; bilateral, both eyes; P, prednisone (oral); TM, transverse myelitis.
At time of sample collection.
Steroid treatment within 1 month before sampling (all patients did not receive maintenance treatment for at least 3 months).
All AQP4-IgG and MOG-IgG serum titres were measured within the same experiment using ΔMFI.
Ex vivo B-cell subset frequencies were compared to age- and gender-matched treatment-naive multiple sclerosis patients (n = 10) as well as healthy controls (n = 20). As a reference group for corticosteroid-treated patients, we included eight clinically isolated syndrome patients treated with methylprednisolone within 1 month before sampling and diagnosed according to the McDonald 2017 criteria. For the NMOSD and MOGAD groups, serum was collected at the same time as peripheral blood mononuclear cells. An NMOSD relapse was defined as a new episode of disease activity at least 3 months separated from the previous disease episode. All patients gave written informed consent, and the study was approved by the medical ethics committee of Erasmus MC.
Total immunoglobulin G ELISA
Immunoglobulin G concentrations in supernatants were determined by ELISA using flat-bottom 96-well half-area plates (Corning, Tewksbury, USA) coated overnight at 4°C with goat anti-human Ig (1 mg/ml; Southern Biotech, Birmingham, USA). Plates were washed with PBS/0.05%Tween-20 to remove unbound antibody and blocked with PBS/5%FCS for 2 h at room temperature. Sample and a human IgG standard (Sigma-Aldrich/Merck, Darmstadt, Germany) were added for 1.5 h at room temperature. Subsequently, plates were washed with PBS/0.05%Tween-20 and bound IgG was detected by peroxidase-conjugated goat anti-human IgG (Thermo Fisher Scientific, Landsmeer, The Netherlands). TMB Substrate (Thermo Fisher Scientific) was used to reveal peroxidase activity. The reaction was stopped with H2SO4 and optical density was measured at 450 nm.
AQP4- and MOG-IgG cell-based assays
For the determination of AQP4- and MOG-IgG levels in sera and culture supernatants, standardized cell-based assays were used as described previously (Ketelslegers et al., 2011; Pelt van et al., 2016a,b). In short, either HEK293T transfectants with EGFP-tagged AQP4-M23 or 1:1 mixtures of LN18 cells transfected with and without full-length human MOG were incubated with the sample and stained with goat anti-human secondary antibody (IgG labelled with APC; Jackson ImmunoResearch, Amsterdam, The Netherlands). Our AQP4-IgG cell-based assays showed a mean transfection efficiency (GFP+) of ∼35%. Mean fluorescence intensity (MFI) representing the amount of AQP4- or MOG-IgG bound to the cell surface was compared between transfected (GFP+) and untransfected (GFP−) cells within the same experiment using flow cytometry.
Cell isolation, antibodies and flow cytometry
Peripheral blood mononuclear cells were collected using Vacutainer CPT® tubes containing sodium heparin according to the manufacturer’s instructions (BD Biosciences, Erembodegem, Belgium). After centrifugation, cells were taken up in RPMI 1640 (Lonza, Basel, Switzerland) containing 40% of fetal calf serum (Lonza) and 20% of dimethyl sulphoxide (Sigma-Aldrich, St. Louis, MO) and stored in liquid nitrogen until further use. Ex vivo naive mature (CD19+CD38dim/−CD27−IgG−IgA−) B cells were purified for in vitro cultures using a BD FACSAria III cell sorter. For immunophenotyping, cells were incubated with Fixable Viability Stain 700 (BD Biosciences) for 15 min and monoclonal antibodies for 30 min at 4°C. The following FACS antibodies were used: CD24 (BV605, ML5), CD27 (BV421, M-T271), IgD (PE-CF594, IA6), IgG (APC-H7, G18-145; BD Biosciences), CD19 (BV785, HIB19), CD38 (PE-Cy7, HIT2), IgM (BV510, MHM-88), T-bet (PE-Cy7, 4B10; Biolegend, London, UK) and IgA (FITC, IS11-8E10; Miltenyi Biotec, Bergisch Gladbach, Germany). For intracellular T-bet staining, cells were fixed with 2% paraformaldehyde (Merck, Schiphol‐Rijk, The Netherlands) and permeabilized using PBS (pH = 7.4) containing 0.3% BSA and 0.5% saponin (Sigma‐Aldrich). All measurements were calculated with an LSRII‐Fortessa flow cytometer and analyzed using FACS Diva software, version 8.0.1 (BD Biosciences). For both ex vivo and in vitro analyses, we first gated on viable CD19+ B cells.
Germinal centre-like B-cell differentiation assay
Germinal centre-like B-cell cultures were performed as described recently (van Langelaar et al., 2019). In short, irradiated murine 3T3 fibroblasts expressing human CD40L were co-cultured with sorted naive mature (CD27−CD38dim/−IgG−IgA−) B cells in the presence of IL-21 (50 ng/ml; Thermo Fisher Scientific) with and without IFN-γ (50 ng/ml; Peprotech, Huissen, The Netherlands) and CpG‐ODN (10 μg/ml; InvivoGen, San Diego, CA, USA). After 11 days of culturing, cells were stained for flow cytometry and supernatants were stored and analyzed for the presence of AQP4- and MOG-IgG.
Statistical analysis
Statistical analysis was performed using Graphpad Prism Software, version 5.04. Kruskal–Wallis and Dunn’s post-hoc tests were performed for comparing multiple groups. Mann–Whitney U-tests were used for comparing two groups. Paired data sets were assessed using Wilcoxon signed-rank tests. Correlations between variables were tested using the Spearman rank or Pearson coefficients, depending on the results of the D’Agostino and Pearson omnibus normality test. Percentages and MFI were displayed as the mean. P-values of <0.05 were considered statistically significant.
Data availability
The raw data that support the here-described findings are available from the corresponding author upon reasonable request.
Results
Naive mature/transitional B-cell ratios are reduced in the blood of treatment-naive AQP4-IgG-positive NMOSD patients
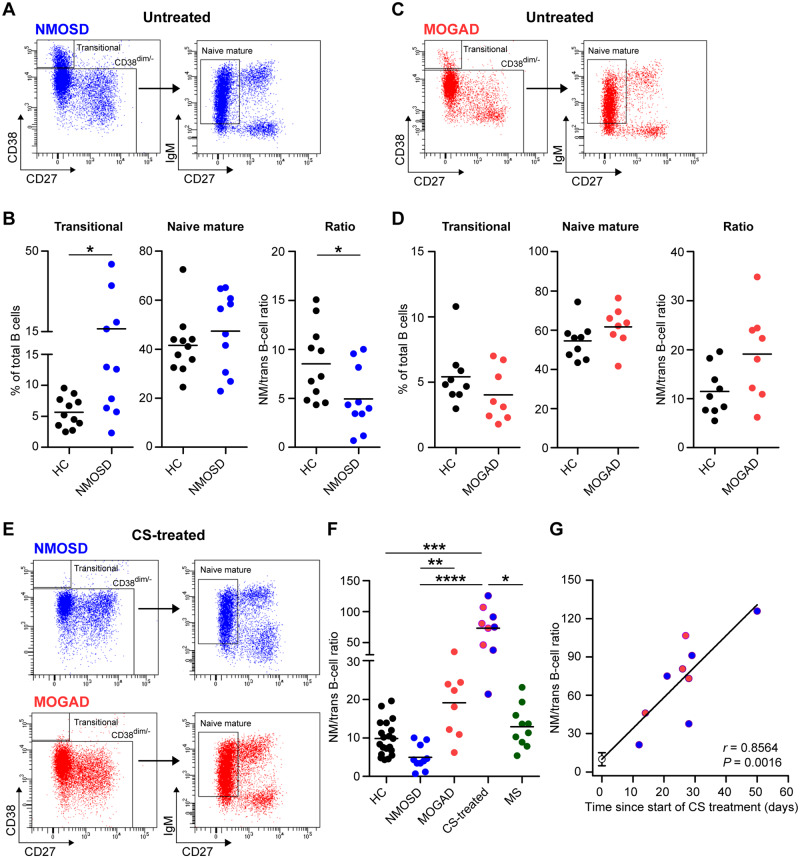
First, we assessed the proportions of naive and memory B-cell subsets in the blood of NMOSD patients without any form of previous immune-suppressive treatment. Because the female-to-male ratio is higher in AQP4- than MOG-IgG-associated disease (Table 1; Pelt van et al., 2016a), we selected healthy controls who were age- and gender-matched either to treatment-naive patients with NMOSD (n = 11) or MOGAD (n = 9). The proportion of transitional B cells (CD38++CD27−) and not naive mature (CD38dim/−CD27−IgM+) B cells (Timmermans et al., 2016; Heeringa et al., 2018) was elevated in NMOSD versus healthy controls (mean 16.2% versus 5.6% for transitional B cells and 47.3% versus 41.5% for naive mature B cells, respectively), which resulted in lowered naive mature/transitional B-cell ratios (Fig. 1A and B). This classification of naive B cells was confirmed using IgD and CD24 (Supplementary Fig. 1). No differences were found between the MOGAD and the healthy control group (Fig. 1C and D). Two out of three NMOSD patients with relapses showed extremely high frequencies of transitional B cells (35% and 44% of the total B-cell pool, Fig. 1B). Naive mature/transitional B-cell ratios were lower in NMOSD compared to MOGAD (P < 0.01; Fig. 1F).
Figure 1.
Transitional and naive mature B-cell frequencies in the blood of different NMOSD, MOGAD, multiple sclerosis and healthy control groups. Representative gating, proportions and ratios of transitional (CD38++CD27−) and naive mature (CD38dim/−CD27−) B cells from blood of treatment-naive patients with NMOSD (n = 10; A, B) or MOGAD (n = 8; C, D). The fractions of transitional and naive mature B cells and their ratios were compared to a separate age- and gender-matched healthy control group (for NMOSD, n = 11; for MOGAD, n = 9). (E) Gating example for the detection of transitional and naive mature B cells in the blood from corticosteroid (CS)-treated patients with NMOSD or MOGAD. (F) Naive mature/transitional B-cell ratios in the blood of treatment-naive NMOSD or MOGAD, CS-treated NMOSD or MOGAD (n = 9), treatment-naive multiple sclerosis (MS; n = 10) and healthy control (HC; n = 20) groups. (G) Correlation of naive mature/transitional B-cell ratios to time since start of CS treatment in patients with NMOSD and MOGAD.
Germinal centre-independent natural effector (CD38dim/−CD27+IgM+IgD+) memory B cells (Berkowska et al., 2011) were significantly reduced in the NMOSD versus healthy control group (lowest in two relapsing cases with the highest percentage of transitional B cells; Supplementary Fig. 2A and B). A similar trend was found in the MOGAD group (Supplementary Fig. 2B). The proportions of germinal centre-dependent IgM-only B cells (CD38dim/−CD27+IgM+IgD−) and IgG+ (both CD27+ and CD27−) B cells (Berkowska et al., 2011) (Supplementary Fig. 2B) or plasmablasts (CD38++CD27++; Supplementary Fig. 3A) did not differ between groups. None of the populations and ratios correlated to AQP4- or MOG-IgG serum levels (Table 1) in the NMOSD groups (data not shown).
Corticosteroid treatment corresponds to increased naive mature/transitional B-cell ratios in AQP4- and MOG-IgG-positive disease
To study the impact of corticosteroids as a standard treatment of acute relapses, we compared our results to B-cell subsets from the blood of nine AQP4-IgG or MOG-IgG-positive patients who received only corticosteroids and no other forms of immunosuppressive treatment. In this group, naive mature/transitional B-cell ratios were significantly elevated (fold change versus NMOSD: 14.8, P < 0.0001; Fig. 1E and F), which correlated positively with time since start of corticosteroid treatment (r = 0.86 and P = 0.002, Fig. 1G). These elevated ratios were the result of an almost complete absence of transitional B cells (Fig. 1E, Supplementary Fig. 4). The association of corticosteroid treatment with transitional and not naive mature B cells was confirmed in an additional cohort of patients with a clinically isolated syndrome (Supplementary Fig. 5). In the corticosteroid-treated NMOSD and MOGAD group, the proportion of natural effector B cells was similar to healthy controls and correlated to time since start of treatment (r = 0.71, P = 0.02; Supplementary Fig. 2C and D). Corticosteroid treatment did not affect plasmablast, IgM-only and IgG+ (CD27−/CD27+) B-cell frequencies (Supplementary Fig. 3B and data not shown).
Toll-like receptor 9 ligand synergizes with IFN-γ to promote naive mature B cell to plasmablast formation only for AQP4- or MOG-IgG-positive patients with relapses
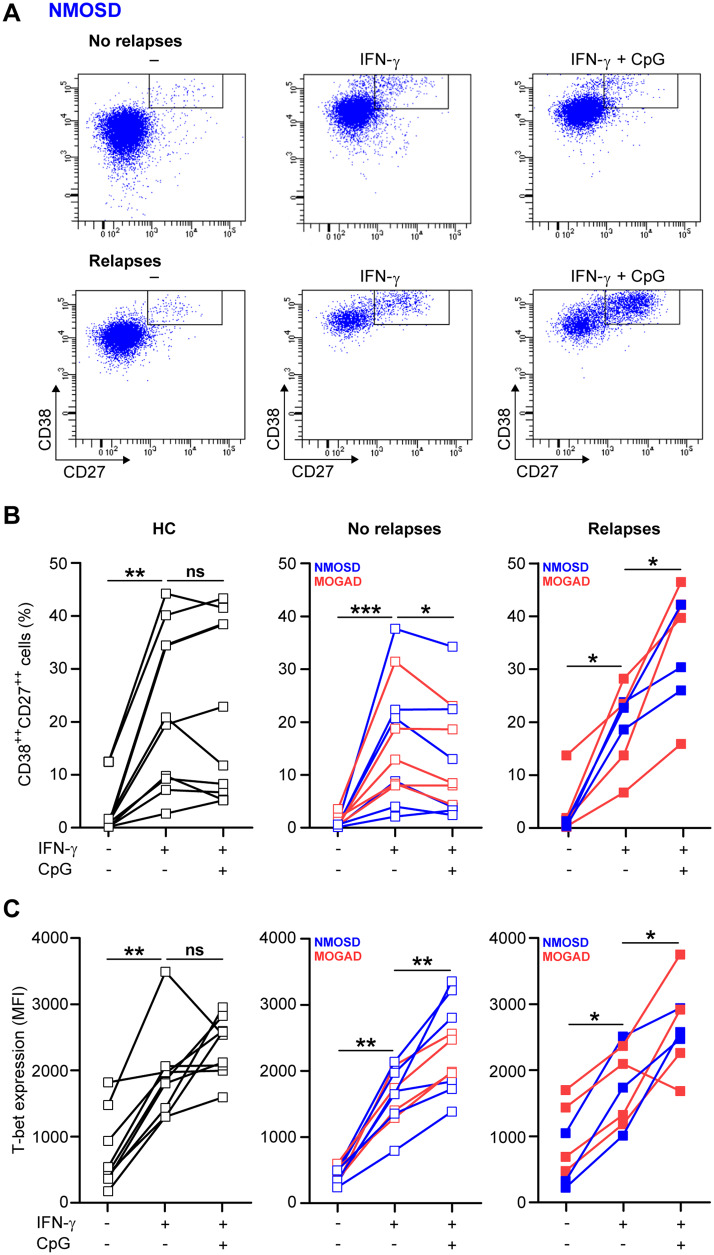
To assess how the B-cell germinal centre-like differentiation pathway is regulated in NMOSD, we purified naive mature B cells from the peripheral blood of nine AQP4-IgG-positive patients, nine MOG-IgG-positive patients and nine healthy controls and cultured these populations in the presence of IL-21 and CD40L-3T3 cells with and without IFN-γ and TLR9 ligand CpG-ODN. The percentage of viable CD38++CD27++ plasmablasts was analysed after 11 days using flow cytometry (Fig. 2A). To further substantiate the clinical relevance of this model, we explored the functional association of in vitro-generated plasmablasts with disease activity within the continuum of relapse risk.
Figure 2.
In vitro plasmablast outgrowth for naive mature B cells from subgroups with and without relapses under different germinal centre-like conditions. (A) Representative gating of viable plasmablasts (CD38++CD27++) cultured from naive mature B cells of an NMOSD patient with and without relapses. Cells were triggered with CD40L-3T3, IL-21, IFN-γ and/or TLR9 ligand CpG-ODN for 11 days. Both the percentage of in vitro-generated plasmablasts (B) and the intracellular T-bet expression (C) were determined for cultured naive mature B cells from the blood of NMOSD or MOGAD subgroups with (n = 7) or without relapses (n = 11), as well as healthy controls (HC; n = 9). For one patient with MOGAD, we obtained only sufficient cell numbers to analyze plasmablast frequencies and not T-bet expression.
For all tested subjects, IFN-γ induced the development of plasmablasts (Fig. 2B), which was similar between the groups. However, in both NMOSD and MOGAD patients with relapses (n = 7), the addition of CpG-ODN to IFN-γ-containing cultures significantly increased plasmablast formation (Fig. 2B; mean, IFN-γ: 19.8%, IFN-γ+CpG: 34.9%, P = 0.0156). The opposite was found in patients without relapses (n = 11; IFN-γ: 15.8%; IFN-γ+CpG: 12.8%, P = 0.0137). Although disease duration was longer for relapsing compared to non-relapsing patients (median 43 versus 17 months, respectively), relapsing patients experienced their first relapse within a median time of 10 months since onset. In six out of seven relapsing patients, immune-suppressive treatment was initiated after the first relapse, whereas six out of 11 monophasic patients were treated from onset onwards. For naive mature B cells of healthy controls, these frequencies were identical for both conditions (IFN-γ: 20.9%, IFN-γ+CpG: 20.4%). Intracellular T-bet levels were up-regulated by IFN-γ and further induced by CpG-ODN (Fig. 2C). Since this was comparable between the groups, other factors probably mediate the observed effect of CpG-ODN on in vitro plasmablast formation. The proportions of ex vivo B-cell subsets and plasmablasts did not differ between the groups with or without relapses (Supplementary Fig. 6).
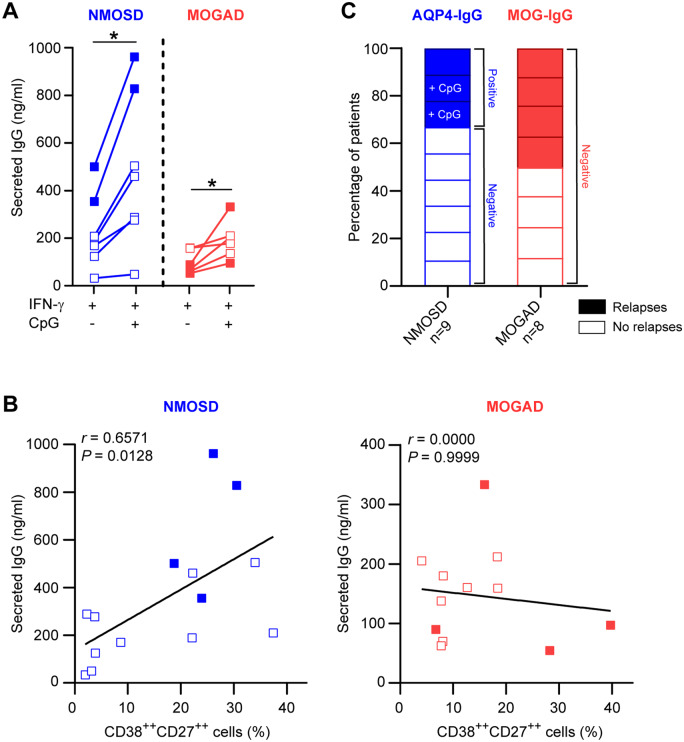
In both the NMOSD and the MOGAD group, in vitro secretion of total IgG was significantly increased after the addition of CpG-ODN (Fig. 3A and Supplementary Fig. 7). For the NMOSD group, this positively correlated with in vitro plasmablast formation (Fig. 3B). The increase in both plasmablast formation and IgG secretion was the most pronounced in the two NMOSD patients with relapses (Fig. 3B). We detected anti-AQP4 IgG in naive mature B-cell culture supernatants of all three relapsing but in none of six non-relapsing patients with NMOSD (Fig. 3C). Anti-AQP4 IgG secretion was enhanced by CpG-ODN for two out of three relapsing patients; for one relapsing case with very high levels in culture (ΔMFI, IFN-γ: 24 442, IFN-γ+CpG: 34 725, negative control: 33) and one relapsing case with very low levels in culture (ΔMFI, IFN-γ: 57, IFN-γ+CpG: 94, negative control: 54). In culture supernatants of naive mature B cells of four relapsing and four non-relapsing patients with MOGAD, anti-MOG IgG could not be detected (Fig. 3C; ΔMFI, positive control: 7322). In this group, total IgG levels did not correlate with in vitro plasmablast formation (Fig. 3B).
Figure 3.
IgG secretion by in vitro-generated plasmablasts using naive mature B cells from subgroups with and without relapses. (A, B) Total IgG secretion was measured in germinal centre-like cultures with naive mature B cells from patients with NMOSD (n = 7) or MOGAD (n = 6; 11 days). In each subgroup, two patients had relapsing disease (solid boxes). Data were compared between IFN-γ- and both IFN-γ- and CpG-ODN-inducing conditions (A) and correlated to in vitro plasmablast (CD38++CD27++) formation (B). (C) Detection of anti-AQP4-IgG and -MOG-IgG in naive mature B-cell culture supernatants (11 days) for patients with NMOSD or MOGAD. Aquaporin-4-IgG levels were elevated in IFN-γ-containing cultures with CpG-ODN for two NMOSD patients with relapses.
Discussion
Aquaporin-4-IgG production by peripheral B cells is an important driver of NMOSD (Hauser et al., 2008; Bar-Or et al., 2010; Kim et al., 2011). Since central B-cell tolerance mechanisms are defective in NMOSD (Kinnunen et al., 2013; Cotzomi et al., 2019), the selection of AQP4-specific B cells is already disturbed at the earliest stage in the bone marrow. We now show that the composition and functional outgrowth of circulating naive B cells is different in patients with NMOSD, which seems to be linked to corticosteroid treatment, relapse occurrence and AQP4-IgG secretion.
The selective enrichment of transitional B cells in the blood of AQP4-IgG-positive NMOSD patients is likely caused by higher fractions of poly- and auto-reactive clones that escaped selection within the bone marrow (Cotzomi et al., 2019). We now find that transitional B cells are almost completely absent in the blood of corticosteroid-treated patients. Since the patients described by Cotzomi et al. (2019) received immunotherapy including corticosteroids, AQP4-specific B cells and other unique clones may have been missed in their analysis. This effect of steroids on transitional B cells is probably explained by their lack of multidrug-resistance receptor 1 (Wirths and Lanzavecchia, 2005), a glycoprotein that pumps a wide range of substances including corticosteroids out of the cell (Brinkmann and Eichelbaum, 2001). Transitional B cells were abrogated by corticosteroid treatment in patients with NMOSD and MOGAD. This correlated to time since start of treatment in clinically isolated syndrome patients as well, implicating that corticosteroids have a generic impact on these early B-cell emigrants.
In a previous study, the proportion of circulating CD27+ memory B cells was found to be reduced in patients with NMOSD (Kowarik et al., 2017). We find that frequencies of germinal centre-independent CD27+IgM+IgD+ memory B cells are lower in patients with NMOSD and recovered after corticosteroid treatment. These data suggest that AQP4-specific naive mature B cells preferentially enter the germinal centre to undergo proliferation and somatic hypermutation. Consistent with this, under germinal centre-like conditions in vitro, AQP4-specific plasmablast development occurred more for naive than memory B cells and did not require antigen (Wilson et al., 2018), and somatic hypermutation was shown to be essential for generating AQP4-specific antibodies (Cotzomi et al., 2019). We did not find any increase in circulating memory B-cell subsets, including those lacking CD27 expression (Kowarik et al., 2017). This implies that within germinal centres, AQP4-specific naive B cells develop into plasmablasts rather than memory B cells. Indeed, some studies showed that ex vivo circulating plasmablasts are expanded in NMOSD, which seemed to be irrespective of steroid usage (Chihara et al., 2011; Kowarik et al., 2017). In this study, no differences in ex vivo CD38++CD27++ plasmablasts were found, similar to the observations by Wilson et al. (2018). The vulnerability of plasmablasts for freeze–thaw cycles (Kyu et al., 2009) can be a confounding factor although this was similar for all patients in this study. Another factor is that plasmablasts probably further mature and reside within the bone marrow or inflamed tissues to produce AQP4-specific antibodies (Bennett et al., 2015; Wilson et al., 2018).
Although the ability of ex vivo plasmablasts to produce AQP4-IgG is highly controversial (Chihara et al., 2011; Wilson et al., 2018), the development of naive B cells into antibody-secreting cells has become widely accepted. In the study by Cotzomi et al. (2019), none of the recombinant anti-AQP4 IgGs reverted back to unmutated precursors were able to bind to AQP4, indicating that naive mature B cells need to enter germinal centres to develop into anti-AQP4 IgG producers. Recently, we found that IFN-γ induces naive mature B cells to develop into plasmablasts during germinal centre-like cultures (van Langelaar et al., 2019). The same is true for naive mature B cells of patients with NMOSD (this study). However, in contrast to patients with multiple sclerosis (van Langelaar et al., 2019), the addition of CpG-ODN had an inducing effect on in vitro plasmablast outgrowth for NMOSD patients with recorded relapses. This difference in in vitro naive mature B-cell outgrowth is likely related to the impaired central B-cell tolerance found in NMOSD and not in multiple sclerosis (Cotzomi et al., 2019). Based on the data in this study, it is tempting to speculate that the elevated frequencies of (auto-reactive) transitional B cells in patients with a high relapse risk causes preferential development of naive mature B cells into AQP4-IgG-secreting plasmablasts within secondary lymphoid organs. However, our findings should be interpreted cautiously due to low patient numbers and inherent differences in the clinical course between NMOSD, MOGAD and multiple sclerosis.
CpG-ODN alone is known to suppress (Jackson et al., 2014), but synergizes with IFN-γ to potentiate auto-reactive T-bet+ B cells (Rivera-Correa et al., 2017). T-bet expression does not seem to mediate the difference in IFN-γ- and CpG-ODN-induced plasmablast induction in vitro between relapsing and non-relapsing NMOSD groups. An intriguing scenario may be that IFN-γ enhances IL-6 production by B cells (Arkatkar et al., 2017), leading to TLR9 up-regulation (Carvalho et al., 2011). Toll-like receptor ligation has been previously associated with the activation of transcription factor X-box-binding protein 1 (Martinon et al., 2010), which enhances the secretion of IgG (Iwakoshi et al., 2003) and links to the IL-6-driven survival of AQP4-IgG-secreting plasmablasts (Chihara et al., 2011).
A limitation of this study is the relatively low sample size. The results obtained here need to be confirmed in longitudinal studies and in additional cohorts. However, to our knowledge, this is the first study that assessed the phenotype and responsiveness of naive B cells of NMOSD patients without immunomodulatory treatment and compared these to MOGAD, multiple sclerosis and healthy control groups. The impact of steroids on transitional but not on naive mature B cells could be a mechanistic explanation why NMOSD relapses recur after discontinuation of steroid treatment. Our findings also provide a rationale for exploring the underlying mechanisms of naive B-cell development in patients with active or stable disease. Further assessment of CpG-ODN-mediated outgrowth of naive B cells under germinal centre-like, IFN-γ-containing in vitro conditions may be a new approach to predict NMOSD relapses.
Supplementary material
Supplementary material is available at Brain Communications online.
Supplementary Material
Acknowledgements
The authors thank all patients and controls for donating their blood. They also thank Ruth Huizinga for sharing her experience with the IgG ELISA. The authors would like to dedicate this manuscript to the memory of Prof. Rogier Q. Hintzen, who passed away on 15 May 2019.
Funding
Dutch Multiple Sclerosis Research Foundation (15-490d MS).
Competing interests
The authors report no competing interests.
Glossary
- AQP4 =
aquaporin-4
- CD40L =
CD40 ligand
- FACS =
fluorescence-activated cell sorting
- HEK =
human embryonic kidney
- IFN-γ =
interferon-gamma
- Ig =
immunoglobulin
- IL =
interleukin
- MOG =
myelin oligodendrocyte glycoprotein
- MOGAD =
MOG-IgG-associated disorder
- MS =
multiple sclerosis
- NMOSD =
neuromyelitis optica spectrum disorder
- T-bet =
T-box transcription factor
- Th =
T helper
- TLR9 =
Toll-like receptor 9
References
- Arkatkar T, Du SW, Jacobs HM, Dam EM, Hou B, Buckner JH, et al. B cell-derived IL-6 initiates spontaneous germinal center formation during systemic autoimmunity. J Exp Med 2017; 214: 3207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Or A, Fawaz L, Fan B, Darlington PJ, Rieger A, Ghorayeb C, et al. Abnormal B-cell cytokine responses a trigger of T-cell-mediated disease in MS? Ann Neurol 2010; 67: 452–61. [DOI] [PubMed] [Google Scholar]
- Bennett JL, O’Connor KC, Bar-Or A, Zamvil SS, Hemmer B, Tedder TF, et al. B lymphocytes in neuromyelitis optica. Neurol Neuroimmunol Neuroinflamm 2015; 2: e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowska MA, Driessen GJ, Bikos V, Grosserichter-Wagener C, Stamatopoulos K, Cerutti A, et al. Human memory B cells originate from three distinct germinal center-dependent and -independent maturation pathways. Blood 2011; 118: 2150–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann U, Eichelbaum M. Polymorphisms in the ABC drug transporter gene MDR1. Pharmacogenomics J 2001; 1: 59–64. [DOI] [PubMed] [Google Scholar]
- Carvalho A, Osório NS, Saraiva M, Cunha C, Almeida AJ, Teixeira-Coelho M, et al. The C allele of rs5743836 polymorphism in the human TLR9 promoter links IL-6 and TLR9 up-regulation and confers increased B-cell proliferation. PLoS One 2011; 6: e28256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chihara N, Aranami T, Sato W, Miyazaki Y, Miyake S, Okamoto T, et al. Interleukin 6 signaling promotes anti-aquaporin 4 autoantibody production from plasmablasts in neuromyelitis optica. Proc Natl Acad Sci USA 2011; 108: 3701–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotzomi E, Stathopoulos P, Lee CS, Ritchie AM, Soltys JN, Delmotte FR, et al. Early B cell tolerance defects in neuromyelitis optica favour anti-AQP4 autoantibody production. Brain 2019; 142: 1598–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid SHM, Whittam D, Mutch K, Linaker S, Solomon T, Das K, et al. What proportion of AQP4-IgG-negative NMO spectrum disorder patients are MOG-IgG positive? A cross sectional study of 132 patients. J Neurol 2017; 264: 2088–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med 2008; 358: 676–88. [DOI] [PubMed] [Google Scholar]
- Heeringa JJ, Fieten KB, Bruins FM, van Hoffen E, Knol EF, Pasmans SGMA, et al. Treatment for moderate to severe atopic dermatitis in alpine and moderate maritime climates differentially affects helper T cells and memory B cells in children. Clin Exp Allergy 2018; 48: 679–90. [DOI] [PubMed] [Google Scholar]
- Iwakoshi NN, Lee AH, Glimcher LH. The X-box binding protein-1 transcription factor is required for plasma cell differentiation and the unfolded protein response. Immunol Rev 2003; 194: 29–38. [DOI] [PubMed] [Google Scholar]
- Jackson SW, Scharping NE, Kolhatkar NS, Khim S, Schwartz MA, Li Q-Z, et al. Opposing impact of B cell-intrinsic TLR7 and TLR9 signals on autoantibody repertoire and systemic inflammation. J Immunol 2014; 192: 4525–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurynczyk M, Messina S, Woodhall MR, Raza N, Everett R, Roca-Fernandez A, et al. Clinical presentation and prognosis in MOG-antibody disease: a UK study. Brain 2017; 140: 3128–38. [DOI] [PubMed] [Google Scholar]
- Ketelslegers IA, Modderman PW, Vennegoor A, Killestein J, Hamann D, Hintzen RQ. Antibodies against aquaporin-4 in neuromyelitis optica: distinction between recurrent and monophasic patients. Mult Scler J 2011; 17: 1527–30. [DOI] [PubMed] [Google Scholar]
- Kim S-H, Kim W, Li XF, Jung I-J, Kim HJ. Repeated treatment with rituximab based on the assessment of peripheral circulating memory B cells in patients with relapsing neuromyelitis optica over 2 years. Arch Neurol 2011; 68: 1412–20. [DOI] [PubMed] [Google Scholar]
- Kinnunen T, Chamberlain N, Morbach H, Cantaert T, Lynch M, Preston-Hurlburt P, et al. Specific peripheral B cell tolerance defects in patients with multiple sclerosis. J Clin Invest 2013; 123: 2737–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitley J, Leite MI, Nakashima I, Waters P, McNeillis B, Brown R, et al. Prognostic factors and disease course in aquaporin-4 antibody-positive patients with neuromyelitis optica spectrum disorder from the United Kingdom and Japan. Brain 2012; 135: 1834–49. [DOI] [PubMed] [Google Scholar]
- Kowarik MC, Astling D, Gasperi C, Wemlinger S, Schumann H, Dzieciatkowska M, et al. CNS aquaporin‐4‐specific B cells connect with multiple B‐cell compartments in neuromyelitis optica spectrum disorder. Ann Clin Transl Neurol 2017; 4: 369–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyu SY, Kobie J, Yang H, Zand MS, Topham DJ, Quataert SA, et al. Frequencies of human influenza-specific antibody secreting cells or plasmablasts post vaccination from fresh and frozen peripheral blood mononuclear cells. J Immunol Methods 2009; 340: 42–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon F, Chen X, Lee AH, Glimcher LH. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat Immunol 2010; 11: 411–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelt van ED, Wong YYM, Ketelslegers IA, Hamann D, Hintzen RQ. Neuromyelitis optica spectrum disorders: comparison of clinical and magnetic resonance imaging characteristics of AQP4-IgG versus MOG-IgG seropositive cases in the Netherlands. Eur J Neurol 2016. a; 23: 580–7. [DOI] [PubMed] [Google Scholar]
- Pelt van ED, Wong YYM, Ketelslegers IA, Siepman DA, Hamann D, Hintzen RQ. Incidence of AQP4-IgG seropositive neuromyelitis optica spectrum disorders in the Netherlands: about one in a million. Mult Scler J Exp Transl Clin 2016. b; 2:205521731562565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Correa J, Guthmiller JJ, Vijay R, Fernandez-Arias C, Pardo-Ruge MA, Gonzalez S, et al. Plasmodium DNA-mediated TLR9 activation of T-bet+ B cells contributes to autoimmune anaemia during malaria. Nat Commun 2017; 8: 1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadoun S, Papadopoulos MC. Aquaporin-4 in brain and spinal cord oedema. Neuroscience 2010; 168: 1036–46. [DOI] [PubMed] [Google Scholar]
- Sabatino JJ, Pröbstel A-K, Zamvil SS. B cells in autoimmune and neurodegenerative central nervous system diseases. Nat Rev Neurosci 2019; 20: 728–45. [DOI] [PubMed] [Google Scholar]
- Shahmohammadi S, Doosti R, Shahmohammadi A, Mohammadianinejad SE, Sahraian MA, Azimi AR, et al. Autoimmune diseases associated with neuromyelitis optica spectrum disorders: a literature review. Mult Scler Relat Disord 2019; 27: 350–63. [DOI] [PubMed] [Google Scholar]
- Sindhava VJ, Oropallo MA, Moody K, Naradikian M, Higdon LE, Zhou L, et al. A TLR9-dependent checkpoint governs B cell responses to DNA-containing antigens. J Clin Investig 2017; 127: 1651–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermans WMC, van Laar JAM, van der Houwen TB, Kamphuis LSJ, Bartol SJW, Lam KH, et al. B-cell dysregulation in Crohn’s disease is partially restored with infliximab therapy. PLoS One 2016; 11: e0160103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Langelaar J, Rijvers L, Janssen M, Wierenga-Wolf AF, Melief M-J, Siepman TA, et al. Induction of brain-infiltrating T-bet-expressing B cells in multiple sclerosis. Ann Neurol 2019; 86: 264–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Wang J, Kumar V, Karnell JL, Naiman B, Gross PS, Autoimmunity Molecular Medicine Team, et al. IL-21 drives expansion and plasma cell differentiation of autoreactive CD11chiT-bet+ B cells in SLE. Nat Commun 2018; 9: 1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R, Makuch M, Kienzler A-K, Varley J, Taylor J, Woodhall M, et al. Condition-dependent generation of aquaporin-4 antibodies from circulating B cells in neuromyelitis optica. Brain 2018; 141: 1063–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015; 85: 177–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol 2007; 6: 805–15. [DOI] [PubMed] [Google Scholar]
- Wirths S, Lanzavecchia A. ABCB1 transporter discriminates human resting naive B cells from cycling transitional and memory B cells. Eur J Immunol 2005; 35: 3433–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data that support the here-described findings are available from the corresponding author upon reasonable request.