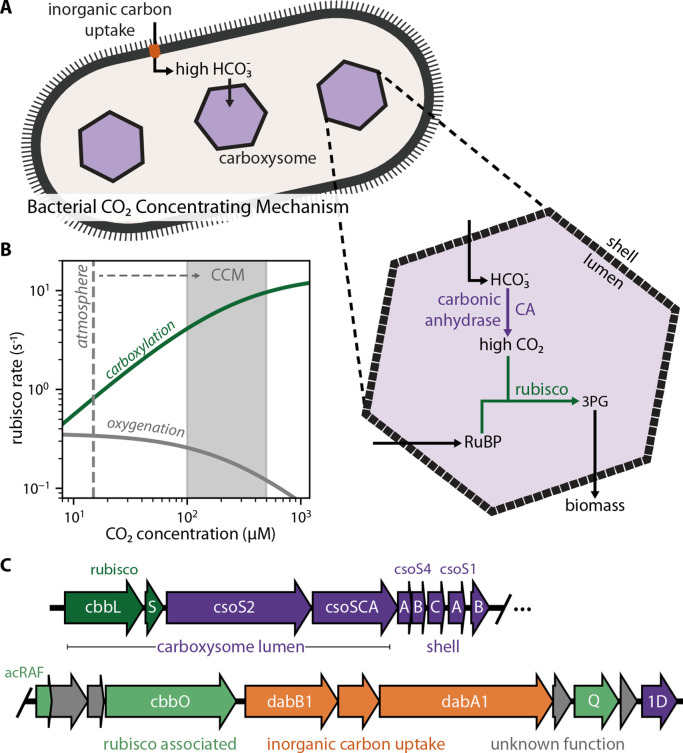
(
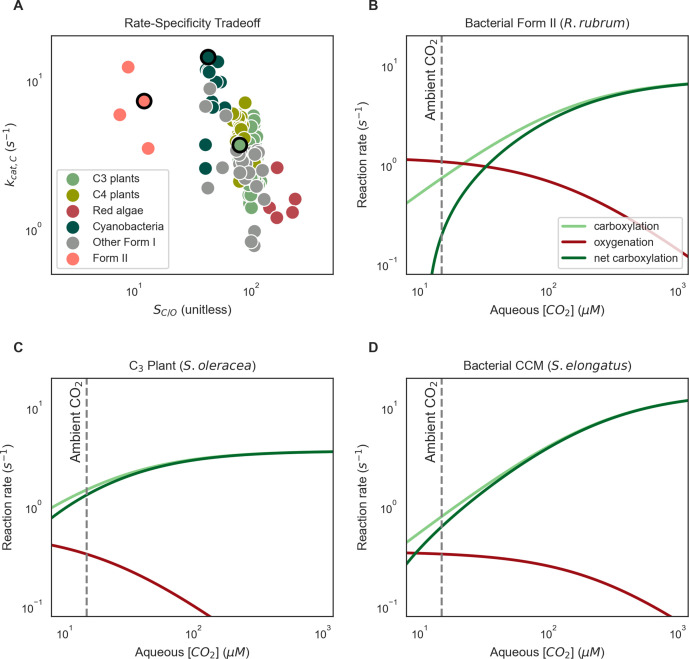
A) Kinetic data assembled for ≈300 rubiscos from diverse organisms show that there is limited variation (less than one order of magnitude) in CO
2 specificity (S
C/O) and maximum carboxylation rate (k
cat,C) among the Form I rubiscos found in all photoautotrophs and all bacteria harboring carboxysome CCMs (
Flamholz et al., 2019). Moreover, S
C/O and k
cat,C appear to trade-off with each other. Although this relationship is not strict, rubiscos with high k
cat,C values also typically have lower S
C/O(
Savir et al., 2010;
Tcherkez et al., 2006). As carboxylation and oxygenation reactions occur at the same active site, elevated CO
2 will both increase the carboxylation rate (until it reaches k
cat,C) and also inhibit oxygenation by exclusion of oxygen from the active site. As it relies only on the well-founded assumption that catalysis with CO
2 and O
2 substrates are mutually exclusive, this mechanism should function for any rubisco. Panels B-D depict this effect for three distinct rubiscos, which are highlighted with black borders in (
A). Panels give carboxylation (light green), oxygenation (red) and net carboxylation (dark green) rates as a function of the aqueous CO
2 concentration at ambient O
2 levels (270 uM at 25 ℃). All curves were calculated using standard kinetic equations for rubisco. Net carboxylation was calculated as the carboxylation rate less ½ the oxygenation rate, which presumes a plant-type photorespiratory pathway that loses one CO
2 for every two oxygenation reactions. (
B) Bacterial Form II rubiscos are typically found in organisms living in low O
2 environments and, accordingly, display low CO
2 specificities (S
C/O ≈ 10) and relatively high maximum carboxylation rates (k
cat,C ≈10–20 s
−1,
Davidi et al., 2020). As such, Form II rubiscos do not perform well in ambient CO
2 and O
2 concentrations. (
C) C
3 plants like spinach do not have CCMs. Furthermore, the CO
2 concentration inside the leaf is typically measured to be lower than ambient due to a balance of stomatal conductance and CO
2 fixation by rubisco itself (
Caemmerer and Evans, 1991). Accordingly, C
3 plant rubiscos display high CO
2 specificities (S
C/O ≈ 100), modest k
cat,C ≈ 3 s
−1, and perform well at ambient and sub-ambient CO
2 levels, displaying relatively little oxygenation and, consequently, net carboxylation rates that are similar to the total carboxylation rate. (
D) Rubiscos found in bacteria with a carboxysome CCM typically have relatively low CO
2 specificities (S
C/O ≈ 50) and fast maximum carboxylation rates relative to other Form I rubiscos (k
cat,C ≈ 10 s
−1). In general, rubiscos from organisms bearing CCMs (whether bacteria, algae, or plants) tend to have lower CO
2 specificities and higher k
cat,C than enzymes from related organisms without CCMs (
Iñiguez et al., 2020;
Savir et al., 2010). The carboxysomal rubisco from
S. elongatus PCC 7942 performs worse than a typical C
3 plant rubisco in ambient air, but much better in the elevated CO
2 environment we presume is maintained by the carboxysome CCM. The aqueous CO
2 and O
2 concentrations were calculated assuming Henry’s law equilibrium at 25 ℃. Notably, changes in temperature will affect CO
2 and O
2 solubility (
Milo and Phillips, 2015;
Sander, 2015) and rubisco kinetics, most notably decreasing CO
2-specificity at elevated temperatures (
Boyd et al., 2019;
Sage et al., 2012).