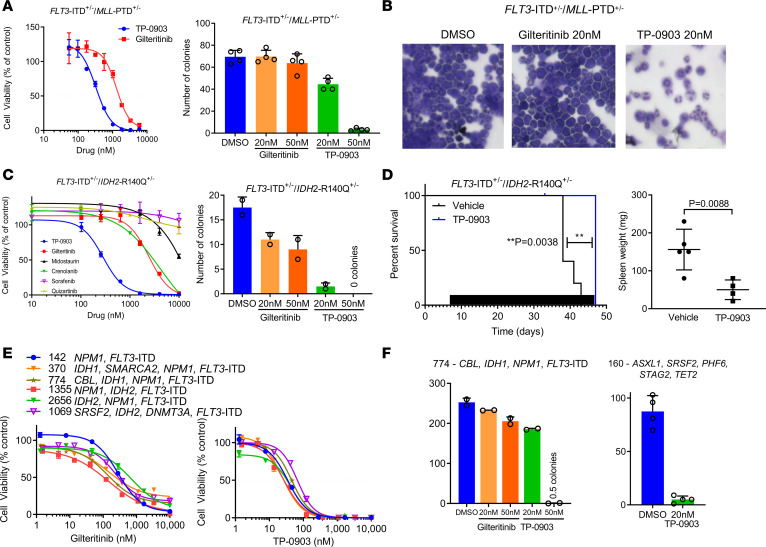
Figure 5. TP-0903 is active in models of AML with recurrent mutations.
(A) Inhibition of cell viability (CellTiterGlo assay, 72 hours, n = 4) (left panel) and colony formation (7 day CFU assay, n = 4) of primary FLT3-ITD/MLL-PTD murine leukemia cells treated ex vivo with indicated TKI. (B) Morphology of treated cells at day 7 by Wright Giemsa staining. Total magnification, 400×. (C) Inhibition of cell viability (CellTiterGlo assay, 72 hours, n = 3–6) and colony formation (7 day CFU assay, n = 4) of primary FLT3-ITD/IDH2-R140Q murine leukemia cells treated ex vivo with indicated TKI. (D) Survival by Kaplan-Meier analysis (left panel) and spleen weight at end of study (right panel) following treatment with TP-0903 (40 mg/kg) once daily or vehicle (n = 5/cohort). Black bars depict days of treatment. (E) Inhibition of viability of human primary AML samples with FLT3-ITD and indicated co-occurring mutations treated with indicated TKI ex vivo (CellTiterGlo assay, 72 hours, n = 3). (F) Inhibition of colony formation of FLT3-ITD+ human primary AML samples treated with indicated TKI (14 day CFU assay, n = 2). P values were determined using either unpaired 2-tailed Student’s t test or log rank test. Specific P values are indicated within the figure.