Figure 1.
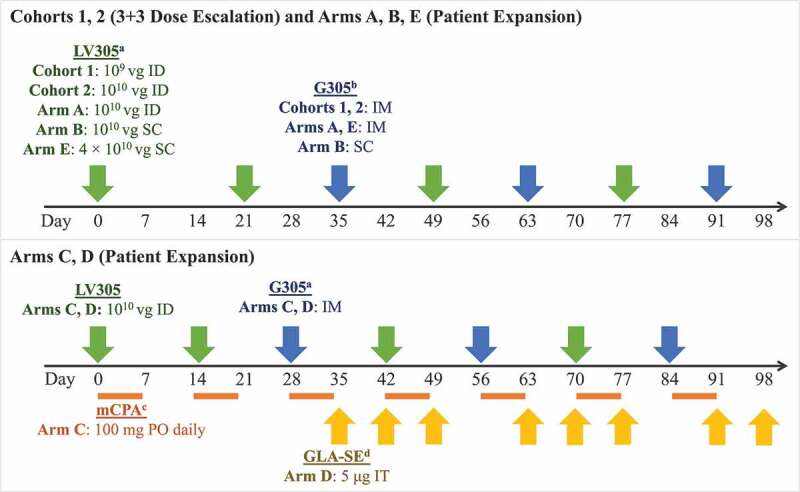
Dose, route, and timing of treatment administration by study arm. a LV305 is a NY-ESO-1 expressing, dendritic-cell tropic lentiviral vector. b G305 is recombinant NY-ESO-1 protein formulated in an oil-in-water stable emulsion with the synthetic TLR4 GLA. G305 dose for all study arms consisted of 250 μg NY-ESO-1 protein mixed with 5-μg GLA-SE. Patients were also given a boosting dose of G305 at each follow-up visit during the first year. c mCPA was only administered to patients in Arm C. It was dosed at 100 mg PO once daily for 7 days, then was not given for the next 7 days, in cycles that repeated until day 97. Patients were given a 1-week supply at each visit.d IT GLA-SE (5 µg/dose) was only administered to patients in Arm D and could have been injected into accessible primary tumors or distant metastases. If no accessible tumor was present at weeks 10, 11, 13, or 14, GLA-SE was not administered. Abbreviations: GLA-SE = glucopyranosyl lipid A-stable emulsion; ID = intradermal; IM = intramuscular; mCPA = metronomic cyclophosphamide; PO = oral; SC = subcutaneous; IT = intratumoral; μg = microgram; vg = viral genomes
