Figure 3.
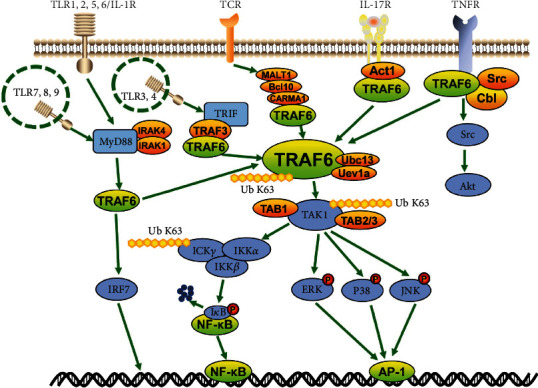
Overall depiction of the TRAF6-mediated signaling pathways. TRAF6 has been identified as a downstream adaptor of multiple receptor families with immunoregulatory functions, including members of the TNFR superfamily, the TLR family, IL-17R, and TCR. These receptors activate TRAF6 by binding directly to TRAF6 or by linking to an intermediate protein. TRAF6 catalyzes the K-63 polyubiquitination of TAK1 by means of Ubc13 and Uev1a. TAK1 forms a complex with TAB1 and TAB2 or TAB3, which phosphorylates and fully activates TAK1. Subsequently, IκB can be phosphorylated and degraded by the activation of the IKK complex, activating NF-κB. However, it can also activate the MAPK pathway, including the ERK pathway, the JNK pathway, and the p38 pathway. In addition, TRAF6 may also directly activate the PI3K and IRF pathways. In this process, TRAF6 conjugates K63-linked polyubiquitin chains (Ub K63) (e.g., IKKβ, TAK1, IRAK, and TRAF6) and K48-linked polyubiquitin chains (Ub K48) (e.g., IκB) to transduce the signal.
