Figure 5.
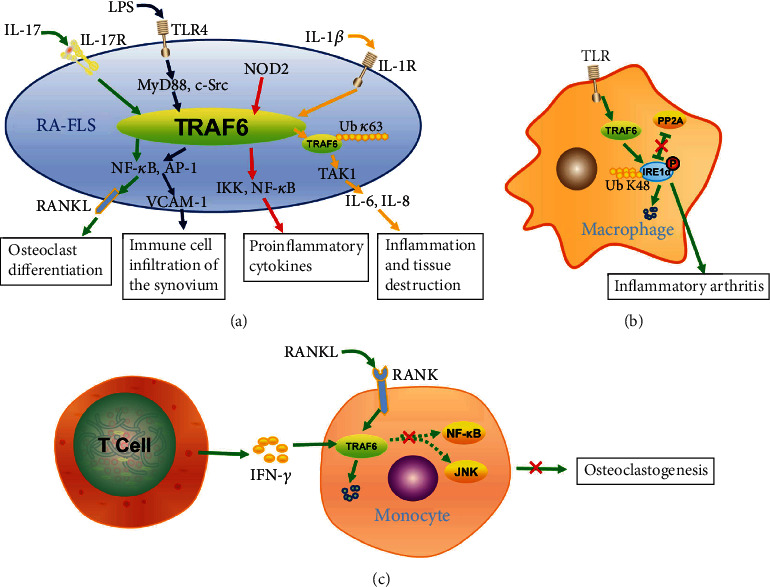
An overview of TRAF6 in the pathogenesis of RA. (a) The role of TRAF6 in RA-FLS on the pathogenesis of RA. LPS regulates NF-κB and AP-1 activation by inducing TLR4/MyD88/TRAF6/c-Src, leading to the induced high expression of VCAM-1 and immune cell infiltration of the synovium. NOD2 protein activates TRAF6, IKK, and NF-κB, leading to the secretion of inflammatory cytokines. In IL-1β-stimulated RA-FLS, the increased autoubiquitination of K63-linked TRAF6 enhances the binding of TRAF6 and TAK1 and induces IL-6 and IL-8 synthesis. IL-17 induces the upregulation of RANKL and osteoclast differentiation mediated by the Act1/TRAF6/NF-κB and AP-1 pathways. (b) IRE1α contributes to the development of inflammatory arthritis. In the early phase of TLR stimulation, the TRAF6-mediated K48-linked polyubiquitin chain leads to the degradation of the IRE1α protein, thereby inhibiting PP2A recruitment, phosphorylating the IRE1α protein, and positively regulating IRE1α activation. In the late phase of stimulation, TRAF6 destroys the IRE1α protein to terminate signal transduction. (c) IFN-γ production by T cell accelerates TRAF6 proteolysis, resulting in the strong inhibition of RANKL-induced NF-κB and JNK activation, and finally inhibiting osteoclastogenesis.
