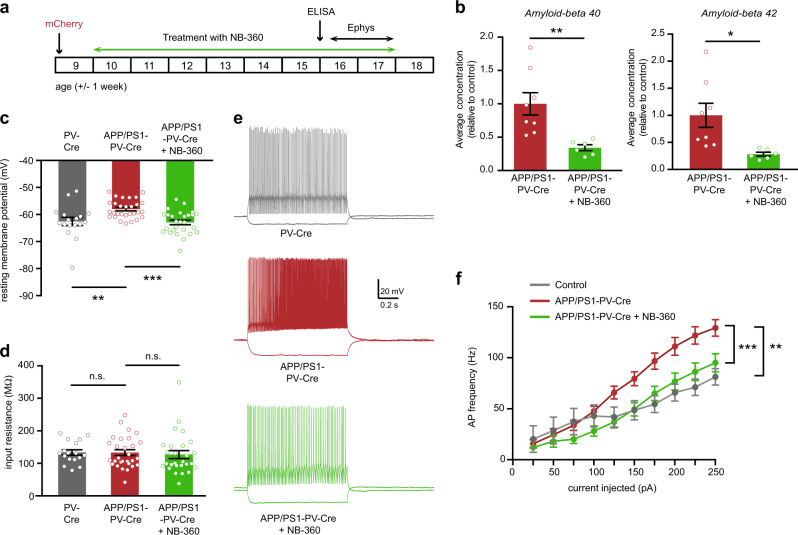
Fig. 3.
Reducing Aβ levels restores PV neuron excitability in APP/PS1 mice in vivo. a Animals were injected with mCherry-expressing virus at 8–10 weeks of age. Mice were then treated with food pellets containing BACE1 inhibitor NB-360 or control food pellets. Mice were sacrificed at 16 weeks of age, after 6 weeks of treatment, for ELISA measurements or at 15–17 weeks of age, after 6–8 weeks of treatment, for electrophysiological recordings. b Levels of Aβ1–40 (left panel) and Aβ1–42 (right panel) in the hippocampus of 16-week-old APP/PS1-PV-Cre that received NB-360 treatment were significantly decreased compared with APP/PS1-PV-Cre mice that received vehicle treatment (Student’s t test; n = 8/6 mice per group, *p < 0.05; **p < 0.01). c PV interneuron resting membrane potential was restored to wild-type levels in NB-360-treated APP/PS1-PV-Cre mice (two-way ANOVA: n = 15/27/28 cells from 3/5/5 mice per group, F2,70 = 9.61, p = 0.0002; post-hoc LSD test: **p < 0.01; ***p < 0.001). d There was no difference in PV interneuron input resistance between groups (two-way ANOVA: n = 15/27/28 cells from 3/5/5 mice per group, F2,70 = 0.20, p = 0.8203). e Voltage responses to 1 s hyperpolarizing or depolarizing current steps from a PV interneuron in wild-type PV-Cre (gray), vehicle-treated APP/PS1-PV-Cre (red), and NB-360-treated APP/PS1-PV-Cre (green) mice. f Average action potential (AP) frequency in response to 0–250 pA depolarizing current steps illustrating a significant rescue of PV interneuron excitability in NB-360-treated APP/PS1-PV-Cre mice compared with vehicle-treated APP/PS1-PV-Cre mice (genotype × current two-way repeated measures ANOVA: 15/27/28 cells from 3/5/5 mice per group, F2,68 = 8.48, p = 0.001; post-hoc LSD test: **p < 0.01; ***p < 0.001)