Abstract
Estrogen-related receptor alpha (ERRα), an orphan nuclear receptor, was reported to be highly associated with the progression and tumorigenesis of several human malignancies. However, the biological role and underlying molecular mechanisms of ERRα in pancreatic cancer (PC) remain unknown. The present study demonstrated that ERRα was significantly overexpressed in PC tissues and cell lines. Its high expression was correlated with tumor size, distant metastasis, TNM stage, tumor differentiation and poor prognosis of PC. Subsequent functional assays showed that ERRα promoted PC cell proliferation, tumor growth, as well as migration and invasion via activating the epithelial-mesenchymal transition. In addition, knockdown of ERRα induced apoptosis and G0/G1 cell cycle arrest in PC cells. Plasminogen activator inhibitor 1 (PAI1) was identified by RNA sequencing, knockdown of which could suppress the cell proliferation, migration and invasion that promoted by ERRα overexpression. Further mechanistic investigation using chromatin immunoprecipitation and dual-luciferase reporter assays revealed that ERRα could bind to the PAI1 promoter region and transcriptionally enhance PAI1 expression. Moreover, our data indicated that ERRα played its oncogenic role in PC via activating the MEK/ERK pathway. Taken together, our study demonstrates that ERRα promotes PC progression by enhancing the transcription of PAI1 and activation of the MEK/ERK pathway, pointing to ERRα as a novel diagnostic and therapeutic target for PC.
Keywords: ERRα, pancreatic cancer, transcription, PAI1, MEK/ERK
Introduction
Pancreatic cancer (PC) is one of the most lethal malignant tumors and the fourth leading cause of cancer-related death worldwide [1]. As opposed to the steady increase in survival rates for most cancers, the 5-year survival rates of PC have not improved significantly in the past decades and remain less than 5% [2-4]. The dismal prognosis of PC is mainly due to its rapid disease progression and highly metastatic potential [5,6]. To date, the diagnosis and treatment of PC relies mostly on imaging methods and curative resection, which are limited and insufficient in many cases [7]. It is reported that most patients with PC are diagnosed at an advanced stage when radical surgery becomes impossible [8,9]. Therefore, uncovering the molecular mechanism governing the process and metastasis of PC as well as identifying novel diagnostic and therapeutic biomarkers are of utmost clinical importance for patients with PC.
Estrogen-related receptor alpha (ERRα) is an orphan nuclear receptor that structurally similar to estrogen receptors, which could interact with estrogen-responsive elements but not with natural estrogens [10,11]. Accumulating evidence indicates that ERRα acts as an oncogenic regulator in the tumorigenesis of a variety of human cancers [12]. For example, the expression of ERRα was aberrantly higher in gallbladder cancer and correlated with poor prognosis [13]. In prostate cancer studies, ERRα has been identified as an important negative prognostic factor [14,15]. However, nothing is currently known regarding the expression and role of ERRα in PC.
In the current study, we performed a series of in vitro and in vivo experiments and revealed that, ERRα was overexpressed in PC tissues and negatively correlated with the prognosis of PC patients. ERRα facilitates PC cell proliferation both in vitro and in vivo, and promotes cell migration and invasion via inducing epithelial-mesenchymal transition (EMT) process. In addition, the knockdown of ERRα induces cell apoptosis and cycle arrest in PC cells. Further mechanistic analysis demonstrated that ERRα plays its oncogenic role in PC by enhancing the transcription of plasminogen activator inhibitor 1 (PAI1, also known as SERPINE1), which activatesthe MEK/ERK signaling pathway. Our study provides the first data that ERRα might be a novel diagnostic and therapeutic target in PC.
Materials and methods
Patient samples and clinicopathologic data
A total of 50 pairs of pancreatic cancer and adjacent non-tumor tissues were obtained from the Department of General Surgery in Xinhua Hospital affiliated with Shanghai Jiao Tong University (Shanghai, China) between 2012 and 2016. All cases hadn’t received any radiotherapy or chemotherapy before surgery, and were histologically confirmed and staged based on the American Joint Committee on Cancer Staging Manual (8th edition). The informed consent was obtained from all enrolled patients and the study was approved by Ethics Committee of Xinhua Hospital Affiliated to Shanghai Jiaotong University School of Medicine (Approval No. XHEC-D-2020-076).
Immunohistochemistry
Immunohistochemistry (IHC) staining was conducted following the standard staining procedure [16]. The ERRα expression level was scored based on the percentage of immunoreactive cells: Negative, <5% immunoreactive cells; Weak, 5%-34% immunoreactive cells; Moderate, 35%-64% immunoreactive cells; Strong, ≥65% immunoreactive cells. The total staining score was based on the sum of the extent and intensity, and samples were classified as negative (0-1), weak (2-3), moderate (4-5) and strong (6-7) staining. Among all the PC tissues, 34 were identified as ERRα-high tissues (moderate and strong staining) and 16 were identified as ERRα-low tissues (negative and weak staining) according to the IHC score.
Cell culture and reagents
Pancreatic cancer cell lines (Mia PaCa-2, PaTu8988, PANC1), normal human pancreatic ductal epithelial cell line (HPNE) and 293T cell line were obtained from the Shanghai Key Laboratory of Biliary Tract Disease Research, Shanghai, China. All cell lines were cultured in high-glucose DMEM (Gibco) supplemented with 10% fetal bovine serum (Gibco), and incubated in a humidified incubator with 5% CO2 at 37°C.
GDC-0994 (HY-15947) was purchased from MedChemExpress and dissolved in dimethylsulfoxide (DMSO). The final DMSO concentration in culture medium was less than 0.1%. Mia PaCa-2 cells were pretreated with 5 μM or 10 μM GDC-0994 for 24 hours before further assays.
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from patient samples and cells using Trizol reagent (Invitrogen). cDNA was synthesized using PrimeScript RT reagent kit with gDNA Eraser (Takara), and the levels of transcripts were detected using SYBR-Green method (Takara) by the StepOnePlus Real-Time thermocycler (Applied Biosystems) following the manufacturer’s instructions. The primers sequences are as follows: ERRα forward, 5’-CACTATGGTGTGGCATCCTG-3’ and ERRα reverse, 5’-CGCTTGGTGATCTCACACTC-3’; PAI1 forward, 5’-ACCGCAACGTGGTTTTCTCA-3’ and PAI1 reverse, 5’-TTGAATCCCATAGCTGCTTGAAT-3’; GAPDH forward, 5’-GGAGCGAGATCCCTCCAAAAT-3’ and GAPDH reverse, 5’-GGCTGTTGTCATACTTCTCATGG-3’.
Cell transfection
ERRα was silenced with small interfering RNAs (siRNAs) using Rfect reagent (BAIDAI) according to the manufacturer’s protocol. The sense sequence are as follows: si-ERRα-1 (sense, GCGAGAGGAGUAUGUUCUA; antisense, UAGAACAUACUCCUCUCGC); si-ERRα-2 (sense, GAGAGGAGUAUGUUCUACUAA; antisense, UUAGUAGAACAUACUCCUCUC); si-PAI1 (sense, UCUCUGCCCUCACCAACAUUC; antisense, GAAUGUUGGUGAGGGCAGAGA). ERK1/2 siRNA was obtained from Cell Signaling Technology (6560s, MA, USA). The shRNA targeting ERRα was synthesized using the si-ERRα-1 sequence and inserted into PGMLV-SC5 vector, and the full-length sequence of ERRα was cloned into pCDNA3.1 vector (Genomeditech, Shanghai). Empty vectors were used as control. Cells were infected by concentrated lentivirus at a multiplicity of infection (MOI) of 90 for 48 hours. Cell lines were selected by puromycin (1 μg/ml) for 1 week to construct stable transfected cell lines, transfection efficiency was verified by qRT-PCR and western blotting.
Immunofluorescence assay
Cells were fixed with 4% paraformaldehyde, and immunofluorescence (IF) assay was conducted using Immunol Fluorence Staining Kit with Cy3 (Beyotime, China) following the manufacturer’s instructions. Briefly, cells were permeabilized in 0.1% Triton-X-100, blocked by 3% BSA, incubated with primary antibody at 4°C overnight and then incubated with Labeled Goat Anti-Rabbit IgG at room temperature away from light. DAPI was used for cell counterstaining. A fluorescence microscope (Leica) was used to capture photos.
Western blot analysis
Protein extraction and western blot was performed according to the standard protocol as previously described [17]. In brief, equal amounts of proteins were separated by SDS-PAGE and transferred onto PVDF membranes. The membranes were blocked with 5% skim milk, incubated with specific primary antibodies at 4°C overnight and then with HRP-conjugated secondary antibody at room temperature for 1 h. Protein signals were detected using a Gel Doc 2000 (Bio-Rad, USA). All primary antibodies were purchased from Cell Signaling Technology.
Cell proliferation assays
Cell Counting Kit-8 (CCK-8) assay and 5-Ethynyl-2’-deoxyuridine (EdU)-488 proliferation assay were performed to evaluate cell proliferation state. For CCK-8 assay, cells were seeded into a 96-well plate at the density of 2000/well with 100 μL complete medium overnight. Each well was then replaced with 10 μL CCK-8 reagent with 90 μL medium, incubated in dark at 37°C for 2 hours and then using SpectraMax 190 Microplate Reader to measure the optical density at 450 nm.
EdU Cell Proliferation Kit (Beyotime) was used to detect cell proliferation according to the manufacturer’s instructions. Cells were incubated with 10 μM EdU in complete medium at 37°C for 2 hours. Azide 488 and Hoechst reagent was used for detection of EdU-positive cells and cell counting, respectively. Images were taken under a fluorescence microscope (Leica).
Colony formation assay
Transfected cells were plated onto 6-well plates (1000 cells/well) and cultured for 7 days. Then cells were fixed with 4% paraformaldehyde for 20 minutes and stained with 0.1% crystal violet stain solution for 15 minutes. Images of the stained plates were photographed and the colonies were counted.
Nude mice xenograft models
Female BALB/c nude mice (4 weeks old, weighted 18-22 g) were purchased from Shanghai Laboratory Animal Center of the Chinese Academy of Sciences. The mice were randomly divided into 4 groups (ERRα, vector, shERRα, vector-shRNA) of 5 mice each and housed under appropriate condition. 2 × 106 PaTu8988 cells with stable transfection of LV-ERRα, LV-shERRα or their empty vectors were injected into the left axilla of each mouse. The volume of tumor was measured with calipers weekly using the following formula: 0.5 × width2 × length. 4 weeks after the cells injection, all mice were sacrificed by cervical dislocation, and the xenograft tumors were carefully dissected and weighed. This animal study was approved by the Ethics Committee of Xinhua Hospital Affiliated to Shanghai Jiaotong University School of Medicine.
Transwell assays
Transwell migration and invasion assays were applied using chamber inserts (Corning) and BioCoat Matrigel Invasion chamber inserts (Corning), respectively. Treated cells were suspended in 200 μL serum-free medium and seeded in the upper chamber. For both assays, the lower chamber was added with 750 μL complete medium. After 24 hours incubation, cells in the lower surface were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet stain solution. 5 random fields of views were photographed under a microscope.
Wound healing assay
Cells were seeded in 6-well plates and cultured to more than 90% cell confluence. Wounds were scratched with same strength by use a 200-μL pipette tip. After washing with fresh medium and incubating for 24 hours, images were taken by a microscope at 5 random fields of views. Wound closure percentage was evaluated by comparing the changes before and after 24 hours.
Apoptosis assay
Annexin V-FITC Apoptosis Detection Kit (Beyotime) was used to detect cell apoptotic ratio following the manufacturer’s instructions. Transfected cells were harvested and resuspended with binding buffer, and stained with 5 μL Annexin V-FITC and 10 μL propidium iodide (PI). After 20 minutes incubation at room temperature away from light, stained cells were analyzed by flow cytometry (BD Biosciences).
Cell cycle analysis
Cell Cycle Analysis Kit (Beyotime) was used for analysis of cell cycle distribution. Transfected cells were collected and fixed with ice-cold 70% ethanol at 4°C overnight. The next day, cells were incubated with RNase A and PI for 30 minutes. DNA content was measured by flow cytometry (BD Biosciences) and analyzed by FlowJo software.
RNA sequencing
Total RNA was extracted from PaTu8988 and PANC1 cells after transfection with si-NC or si-ERRα-1, and then subjected to mRNA sequencing on the BGISEQ-500 platform (Beijing Genomics Institute, China). Of all the differentially expressed genes, 40 most up-regulated genes and 40 most down-regulated genes were selected and analyzed using the BGI online analysis system.
Chromatin immunoprecipitation
The promoter sequence of PAI1 was obtained from NCBI (https://www.ncbi.nlm.nih.gov/), the potential binding sites of ERRα in the promoter region of PAI1 were identified by JASPAR (http://jaspar.genereg.net/). Chromatin immunoprecipitation (ChIP) was performed using the PaTu8988 cells and a ChIP Assay Kit (Beyotime) according to the manufacturer’s protocol. Briefly, cross-linked chromatin was sonicated into 200- to 1000-bp fragments, and then immunoprecipitated with normal rabbit IgG antibody (Cell Signaling Technology) or anti-ERRα antibody (Cell Signaling Technology). Precipitated ChIP samples were analyzed by 2% agarose gel DNA electrophoresis and quantitative PCR (qPCR). Primers for the 2 binding sites are as follows: site 1 forward, 5’-CTCCAACCTCAGCCAGACAA-3’ and site 1 reverse, 5’-CCTCCGATGATACACGGCTG-3’; site 2 forward, 5’-CTCCACAGTGACCTGGTTCG-3’ and site 2 reverse, 5’-CGGGTGACCCAAAAAGCCTA-3’.
Dual-luciferase reporter gene assay
293T cells transfected with full-length ERRα, shERRα or their empty vectors were seeded onto 24-well plates and co-transfected with indicated luciferase reporters. pGL3-PAI1-WT-luc (containing the -2000 bp to -1 bp promoter sequence of PAI1) and pGL3-PAI1-MUT-luc (the 2 binding sites of ERRα in the promoter region of PAI1 were mutated) were synthesized by Genomeditech. At 48 hours post-transfection, luciferase activities were determined by a Dual-Luciferase Reporter Assay System (Promega) following the manufacturer’s instructions. The relative luciferase activities were presented after normalization to Renilla luciferase activity.
Co-immunoprecipitation (Co-IP) assay
Briefly, pretreated cells were isolated and then incubated with anti-PAI1, anti-ERK1/2 or IgG plus Protein A+G agarose beads (Beyotime) at 4°C overnight with gentle rotation. After extensive washing, the samples were carried out for western blot analysis for detection of potential interacting proteins.
Statistical analysis
All experiments were repeated at least three times. Data were analyzed using Prism 8 software and presented as the mean ± standard deviations (SD). The Student’s t test was performed for the comparisons between groups, Pearson chi-square test was used to analyze the correlation between ERRα expression and clinicopathologic variables. Survival analysis was applied by Kaplan-Meier method and log-rank test. P<0.05 was considered statistically significant.
Results
Up-regulated expression of ERRα is associated with poor prognosis in human PC
To identify the biological role of ERRα in PC, we first performed qRT-PCR to detect the mRNA levels of ERRα in 50 pairs of PC tissues and adjacent non-tumor tissues. As shown in Figure 1A, 1B, ERRα mRNA expression was significantly higher in PC tissues than in adjacent non-tumor tissues. We also conducted IHC assay to compare the protein expression of ERRα in these samples (Figure 1D). The results of IHC were consistent with that of qRT-PCR to suggest an up-regulation of ERRα in PC tissues at the protein level (Figure 1E). Then we determined the relationship between ERRα and clinical features in PC patients. As shown in Table 1, high ERRα expression was associated with tumor size (P=0.0065), distant metastasis (P=0.0026), TNM stage (P=0.0021) and tumor differentiation (P=0.0045). Moreover, Kaplan-Meier analysis of overall survival (OS) revealed that upregulated ERRα expression was correlated with poor prognosis in PC (P<0.0001, Figure 1C).
Figure 1.
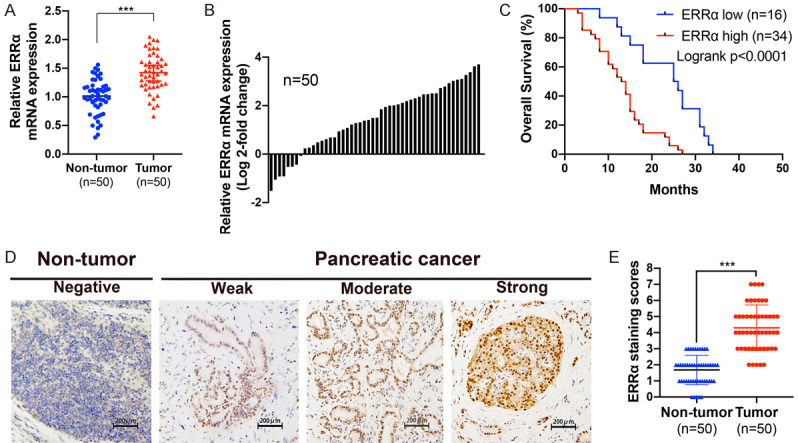
ERRα is overexpressed and correlates with poor prognosis in PC. A. ERRα expression was determined by qRT-PCR in 50 paired PC tissues and adjacent non-tumor tissues. B. The comparisons of ERRα mRNA expression level between PC tissues and adjacent non-tumor tissues. The results were presented as log 2-fold change of tumor tissues relative to non-tumor tissues. C. Overall survival curves of PC patients stratified by ERRα expression. D. Representative images of ERRα expression in PC tissues and adjacent non-tumor tissues using IHC staining. E. The IHC staining scores of ERRα were higher in PC tissues than in adjacent non-tumor tissues. *P<0.05, **P<0.01, ***P<0.001.
Table 1.
Correlation between ERRα expression and clinicopathological characteristics in 50 PC patients
| Parameters | Number of cases | ERRα expression | p-value (χ2-test) | |
|---|---|---|---|---|
|
| ||||
| Low | High | |||
| Gender | NS | |||
| Male | 29 | 9 | 20 | |
| Female | 21 | 7 | 14 | |
| Age (years) | NS | |||
| <60 | 28 | 9 | 19 | |
| ≥60 | 22 | 7 | 15 | |
| Location | NS | |||
| Head, urinate | 22 | 8 | 14 | |
| Body, tail | 28 | 8 | 20 | |
| Tumor Size (cm) | 0.0065** | |||
| <2 | 30 | 14 | 16 | |
| ≥2 | 20 | 2 | 18 | |
| Lymphatic invasion | NS | |||
| Positive | 21 | 6 | 15 | |
| Negative | 29 | 10 | 19 | |
| Distant metastasis | 0.0026** | |||
| Positive | 18 | 1 | 17 | |
| Negative | 32 | 15 | 17 | |
| TNM Stage | 0.0021** | |||
| I-II | 28 | 14 | 14 | |
| III-IV | 22 | 2 | 20 | |
| Tumor differentiation | 0.0045** | |||
| High/Moderate | 26 | 13 | 13 | |
| Poor | 24 | 3 | 21 | |
P<0.01;
NS, non-significant.
ERRα expression in PC cell lines
We performed IF assay, qRT-PCR and western blot assay to detect the expression of ERRα in PC cell lines (PaTu8988, PANC1 and Mia PaCa-2) and HPNE. The results revealed that ERRα was expressed in the nuclei of cells and overexpressed in PC cell lines compared with HPNE (Figure 2A-C). To investigate further, we knocked down ERRα in PaTu8988 and PANC1 cells using siRNAs and overexpressed ERRα in Mia PaCa-2 cells. The transfection efficiency was validated by qRT-PCR and western blot analysis (Figure 2D).
Figure 2.
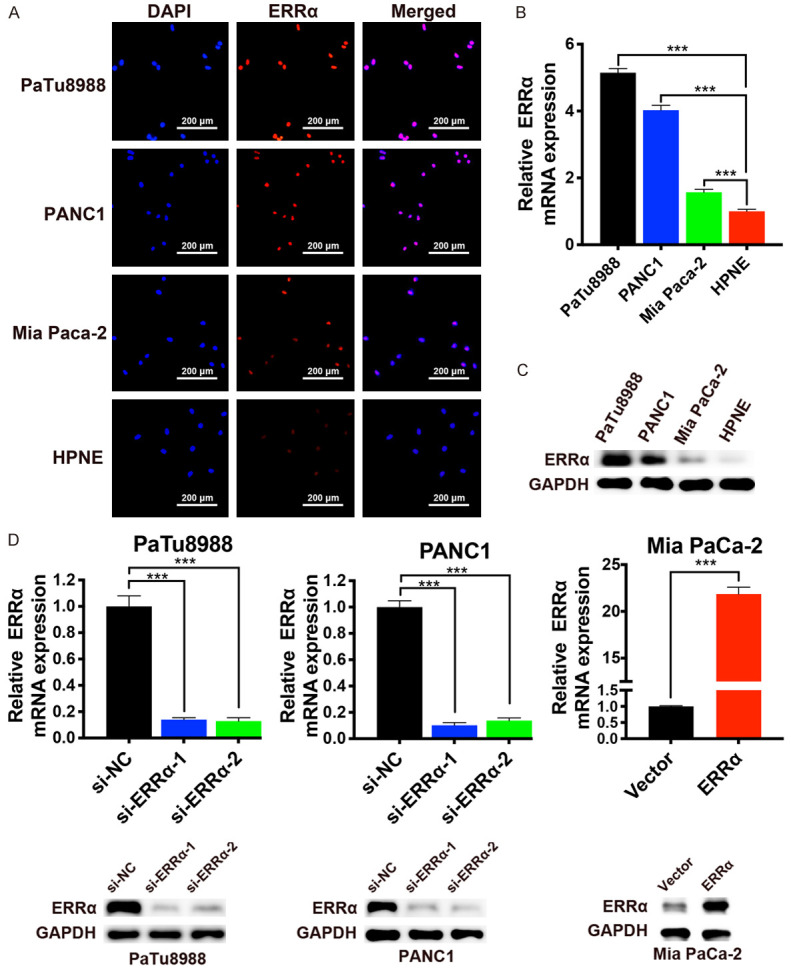
The expression of ERRα in PC cell lines. A. Immunofluorescence staining of ERRα (red) in PC cell lines and normal human pancreatic ductal epithelial cell line (HPNE). Nucleus was stained by DAPI (blue). B and C. The mRNA and protein expression levels of ERRα in PC cell lines and HPNE. D. Knockdown of ERRα in PaTu8988 and PANC1 cells, and overexpression of ERRα in Mia PaCa-2 cells was confirmed at the mRNA and protein level by qRT-PCR and western blotting. *P<0.05, **P<0.01, ***P<0.001.
ERRα promotes PC proliferation in vitro and in vivo
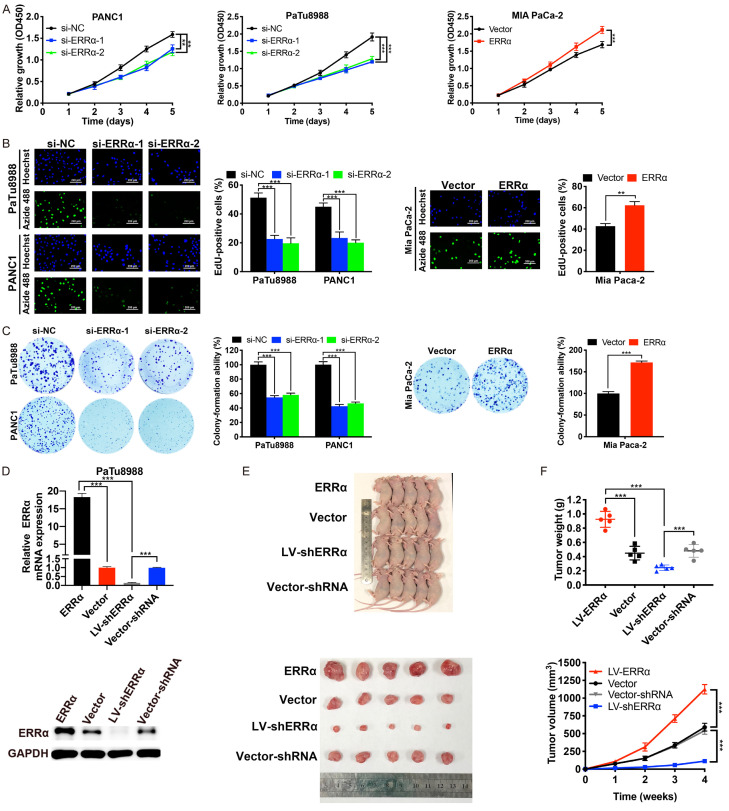
To explore the effects of ERRα on PC cell proliferation in vitro, we conducted CCK-8 assay, EdU-488 proliferation assay and colony formation assay. As shown in Figure 3A-C, the proliferation ability of PC cells was markedly suppressed after ERRα depletion, whereas was enhanced after ERRα overexpression. We also investigated the effect of ERRα on PC tumor growth in vivo. We used PaTu8988 cells with stable transfection of LV-ERRα, LV-shERRα or their empty vectors (Figure 3D) to construct xenograft models. As indicated by the volume, weight and growth curves of the tumors, ERRα overexpression significantly promoted the growth of PaTu8988 cells in xenograft models, whereas ERRα silencing did the opposite (Figure 3E and 3F). These results indicated a growth-promoting role of ERRα on PC in vitro and in vivo.
Figure 3.
ERRα promotes PC cell proliferation and tumor growth. A-C. CCK-8, EdU and colony formation assays were applied to determine the proliferation of PaTu8988 and PANC1 cells after ERRα depletion, and that of Mia PaCa-2 cells after ERRα overexpression. D. The mRNA and protein expression level of ERRα in PaTu8988 cells transfected with LV-ERRα, LV-shERRα or their empty vectors. E. Compared with empty vector groups, overexpression of ERRα promoted while knockdown of ERRα suppressed the tumor growth in nude mice. F. Tumor weight was measured after mice sacrificed and tumor resection; Tumor growth curves were plotted. *P<0.05, **P<0.01, ***P<0.001.
ERRα facilitates PC cell migration and invasion through EMT
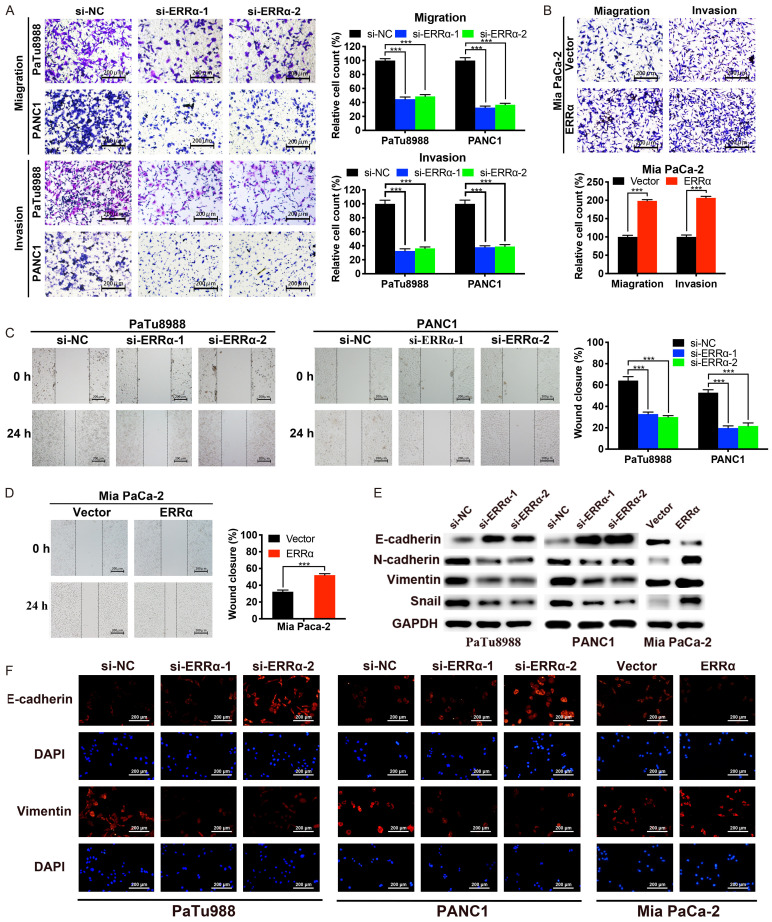
To determine the effect of ERRα on PC metastasis, we assessed PC cell migration and invasion abilities in vitro using wound healing assay as well as transwell migration and invasion assays. As shown in Figure 4A-D, ERRα depletion notably suppressed the migration and invasion ability of PaTu8988 and PANC1 cells compared with negative controls. Conversely, ERRα overexpression remarkably enhanced the cell migration and invasion in Mia PaCa-2 cells. As the EMT process played a vital role in the cancer metastasis [18], we explored whether EMT was involved in the effect of ERRα on migration and invasion. As shown in Figure 4E and 4F, knockdown of ERRα significantly increased the expression levels of epithelial marker E-cadherin, but decreased the expression levels of mesenchymal markers N-cadherin, Vimentin and Snail. What’s more, the overexpression of ERRα presented exactly the opposite results. Collectively, these results indicated that ERRα promotes the ability of migration and invasion via inducing EMT in PC cells.
Figure 4.
ERRα enhances the migration and invasion of PC cells. A and B. Transwell assays were conducted to measure the migration and invasion capabilities of PC cells. C and D. Wound healing assays were used to further determine the migration abilities of PC cells. E. The protein expression levels of EMT related molecules were detected by western blotting. F. The expression levels of E-cadherin and vimentin were assessed by IF assay. *P<0.05, **P<0.01, ***P<0.001.
Knockdown of ERRα induces cell apoptosis and cycle arrest in PC cells
To assess whether the proliferation defects observed in ERRα-depleted PC cells were due to the cell apoptosis and arrest of cell cycle progression, we performed flow cytometry assays to evaluate the effects of ERRα knockdown on PC cell apoptosis and cell cycle regulation. The results demonstrated that ERRα silencing significantly increased the apoptosis rate of PC cells, and the analysis of cell cycle distribution showed that the proportion of G0/G1 phase cells was remarkably increased and that of S-phase cells was decreased after ERRα knockdown (Figure 5A and 5C). In addition, we detected the expression of apoptosis-related and cell cycle-related markers by western blotting and IF assay (Figure 5B, 5D and 5E). The data revealed that protein expression levels of cleaved-PARP, cleaved-caspase 3, cytochrome c, p21 Waf1/Cip1 and p27 Kip1 were significantly increased while that of bcl-2, CDK2, CDK4 and cyclin D1 were decreased after ERRα knockdown. Moreover, ERRα overexpression led to diametrically opposed results. Taken together, the above results provide more insight into the mechanism and indicate that knockdown of ERRα inhibits PC cell proliferation by inducing apoptosis and arrest of the G0/G1 phase.
Figure 5.
Knockdown of ERRα induces cell apoptosis and G0/G1 arrest in PC cells. A. Flow cytometric analysis of apoptosis in PaTu8988 and PANC1 cell after ERRα knockdown. B. Western blot analysis of apoptosis related markers. C. After silencing of ERRα, flow cytometry showed that cell cycles of PaTu8988 and PANC1 cells were arrested at G0/G1 phase. D. Cell cycle related proteins were analyzed by western blotting. E. The expression levels of cleaved-PARP, cleaved-caspase 3, cyclin D1 and p27 Kip1 were detected by IF assay. *P<0.05, **P<0.01, ***P<0.001.
PAI is upregulated and correlated with poor prognosis in PC
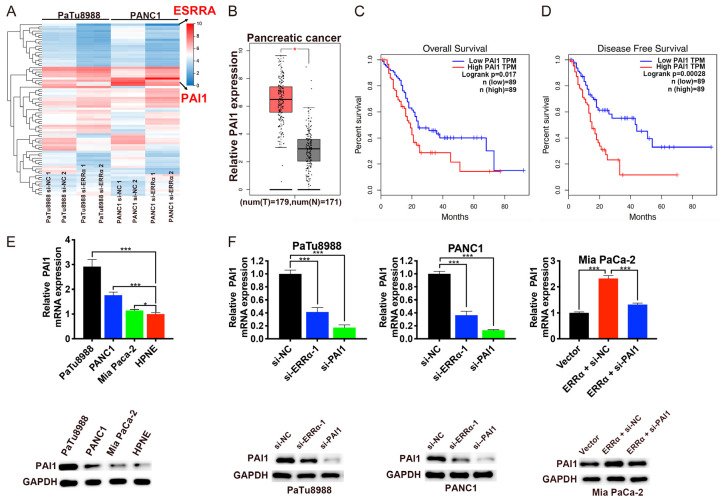
RNA-sequencing was performed to further explore the potential mechanism by which ERRα affects pancreatic cancer progression. Compared with the negative control cells, we selected the top 40 down-regulated and top 40 up-regulated genes in PaTu8988 and PANC1 cells after ERRα knockdown, among which the plasminogen activator inhibitor 1 (fold change =-1.600, P<0.001) attracted our attention (Figure 6A). PAI1, a serine protease inhibitor, has been reported to be involved in the development and progression of several cancers [19-21], however, little is known about the role of PAI1 during tumorigenesis of PC. Based on the GEPIA database, we discovered that the expression level of PAI1 was significantly higher in PC tissues compared with normal tissues (Figure 6B). What’s more, the survival analysis indicated that PC patients with higher PAI1 expression had shorter OS (P=0.017, Figure 6C) and disease free survival (DFS, P=0.00028, Figure 6D). Then the expression levels of PAI1 in PC cell lines and HPNE cell line were determined by qRT-PCR and western blotting. The results demonstrated that PAI1 was upregulated in PC cell lines compared with HPNE cells (Figure 6E). In addition, the expression levels of PAI1 could be decreased by transfection with si-ERRα-1 or si-PAI1 and increased by ERRα overexpression (Figure 6F).
Figure 6.
Upregulated PAI1 correlates with poor prognosis in PC. A. Expression heatmap of top 40 down-regulated and top 40 up-regulated genes after the RNA sequencing analysis following ERRα knockdown in PaTu8988 and PANC1 cells. B. The mRNA expression levels of PAI1 in PC tissues and normal tissues according to GEPIA database. C and D. OS and DFS rate was analyzed in PC patients using GEPIA tool based on PAI1 expression. E. The mRNA and protein levels of PAI1 in PC cell lines and HPNE. F. Expression of PAI1 was decreased in PaTu8988 and PANC1 cells after transfection with si-ERRα-1 or si-PAI1, whereas was increased by ERRα overexpression and could be reversed by PAI1 knockdown. *P<0.05, **P<0.01, ***P<0.001.
PAI1 mediates the oncogenic behavior of ERRα in PC cells
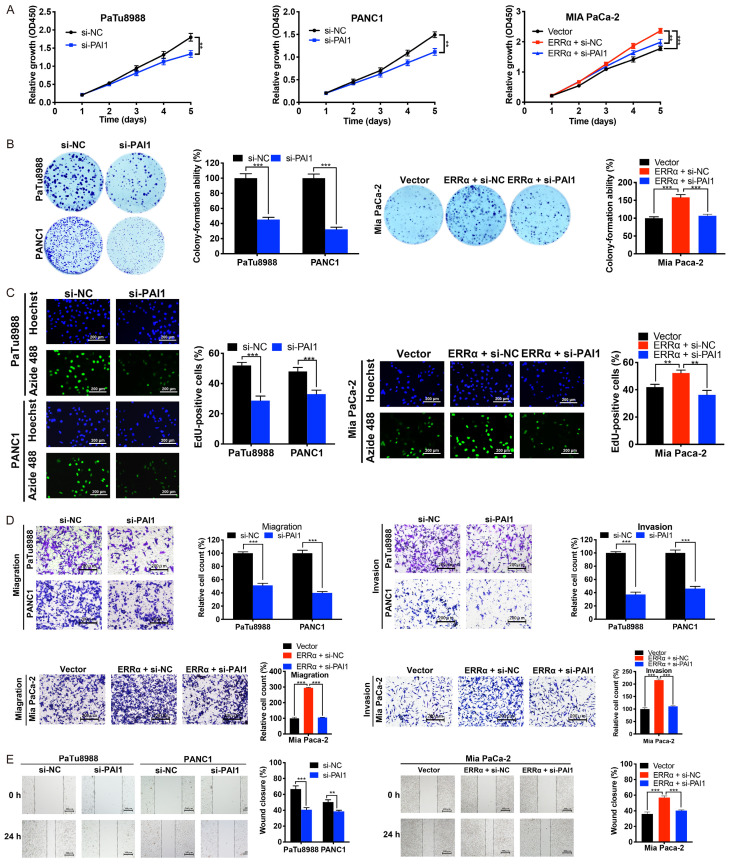
We then investigated whether PAI1 is involved in ERRα-mediated oncogenic effects on PC cells. As shown in Figure 7A-E, the knockdown of PAI1 remarkably inhibited the cell proliferation, migration and invasion in PaTu8988 and PANC1 cells. More importantly, PAI1 depletion could rescue the cell proliferation, migration and invasion enhanced by ERRα overexpression in Mia PaCa-2 cells. Therefore, it is hypothesized that PAI1 participates in the ERRα-induced oncogenic effects in PC.
Figure 7.
PAI1 is involved in ERRα regulated cell proliferation, migration and invasion of PC cells. A-C. CCK-8, colony formation and EdU assays were performed to evaluate the cell proliferation of PC cells with PAI1 knockdown and/or ERRα overexpression. D and E. Knockdown of PAI1 significantly inhibited the migration and invasion abilities of PC cells, and abolished those abilities enhanced by ERRα overexpression. *P<0.05, **P<0.01, ***P<0.001.
ERRα enhances the transcription of PAI1 via directly binding to its promoter
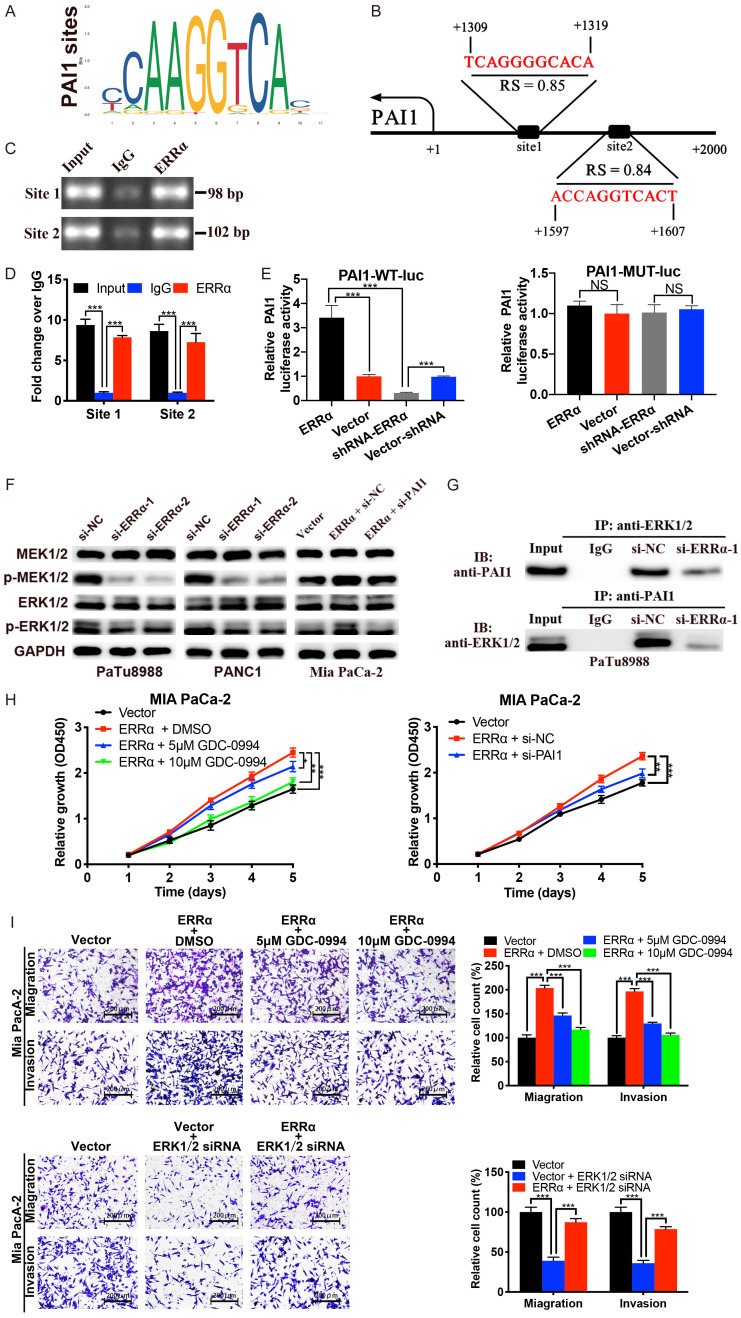
We then investigated the mechanisms responsible for ERRα regulated PAI1. Bioinformatic analysis was performed using JASPAR database and 2 high-scoring predicted binding sites of ERRα in the PAI1 promoter sequence were found (Figure 8A and 8B). ChIP assays were conducted in PaTu8988 cells to confirm the bounding relationship between ERRα protein and PAI1 promoter. ChIP samples were further analyzed via DNA electrophoresis and qPCR. The results of DNA electrophoresis showed the predicted DNA band in Input and ERRα groups but not in the IgG group (Figure 8C), and the results of qPCR confirmed the enrichment of PAI1 promoter in ERRα precipitates (Figure 8D).
Figure 8.
ERRα promotes the transcription of PAI1 and activates the MEK/ERK pathway. A and B. ERRα binding motif and the predicted ERRα binding sites within the promoter region of PAI1 according to the JASPAR database. C and D. ChIP-DNA electrophoresis and ChIP-qPCR using primers designed for the 2 predicted sites validated the binding capacity of ERRα to the PAI1 promoter. E. Dual-luciferase reporter assay showed that ERRα overexpression significantly increased while ERRα knockdown suppressed the activity of the wild-type PAI1 promoter luciferase reporter. Moreover, ERRα had no effect on the activity of mutant PAI1 promoter luciferase reporter. F. The expression levels of p-MEK1/2 and p-ERK1/2 were suppressed by ERRα knockdown; ERRα overexpression enhanced the expression of p-MEK1/2 and p-ERK1/2, which was abolished by knockdown of PAI1. G. Co-IP experiments were performed to detect the interaction between PAI1 and ERK1/2. H and I. The cell proliferation, migration and invasion promoted by ERRα overexpression was significantly antagonized by ERK1/2 inhibitor GDC-0994 and ERK1/2 siRNA.
Subsequent dual luciferase assays demonstrated that the luciferase activity of WT PAI1 promoter reporter was notably increased by ERRα overexpression and decreased by ERRα inhibition. What’s more, the luciferase activity of MUT PAI1 promoter was unaffected by ERRα (Figure 8E). Together, these data indicated that ERRα could bind to the promoter region of PAI1 and transcriptionally enhance the expression of PAI1.
ERRα promotes PC progression through the MEK/ERK signaling pathway
Previous studies have reported that ERRα is associated with the PI3K/AKT pathway [22,23], and the MEK/ERK pathway has crosstalk with PI3K/AKT pathway and plays a vital role in PC [24,25]. Therefore, we investigated whether MEK/ERK signaling pathway was responsible for the oncogenic effects of ERRα in PC. As shown in Figure 8F, the expression levels of p-MEK1/2 and p-ERK1/2 were significantly decreased by ERRα knockdown and increased by ERRα overexpression. What’s more, silencing of PAI1 could attenuate ERRα-induced phosphorylation level of MEK/ERK. Based on the RNA-sequencing results, ERRα didn’t affect the mRNA levels of MEK/ERK, moreover, PAI1 was reported to be able to activate ERK signaling [19]. Co-IP was further performed to examine the potential interaction of PAI1 and ERK1/2, (Figure 8G). The results showed that PAI1 and ERK1/2 could directly interact with each other, and the effect was weakened by downregulation of ERRα. To further determine whether MEK/ERK signaling pathway is critical for the function of ERRα, rescue experiments were conducted using the ERK inhibitor GDC-0994 and ERK1/2 siRNA. CCK-8 and transwell assays revealed that GDC-0994 and ERK1/2 siRNA attenuated the cell proliferation, migration and invasion enhanced by ERRα overexpression (Figure 8H and 8I). Collectively, these results indicated that PAI1 was involved in ERRα-induced MEK/ERK activation in PC, which might be a novel mechanism related to ERRα-mediated regulation of PC development.
Discussion
Accumulating evidence has confirmed the aberrant expression of ERRα in human cancers, and increasing attention has been focused on the vital role of ERRα in the processes related to cancer progression, such as tumor growth, metastasis and chemoresistance [14,16,26,27]. In the present study, we demonstrated that ERRα was extensively upregulated in PC tissues, and its high expression was associated with poor prognosis and several clinicopathological characteristics, such as tumor size, distant metastasis, TNM stage and tumor differentiation in PC patients. Subsequently, a series of in vitro and in vivo assays were conducted and the results showed that, ERRα promoted PC cell proliferation, migration, invasion as well as induced cell apoptosis and G0/G1 cycle arrest via enhancing the transcription of PAI and activating the MEK/ERK signaling pathway. To our knowledge, this is the first study to identify the critical role of ERRα in PC progression, suggesting that ERRα may be a novel diagnostic and therapeutic target for PC.
The present study revealed that ERRα was highly expressed in PC cell lines and knockdown of ERRα could suppress the proliferation and colony formation of PC cells. In addition, EdU proliferation assays demonstrated that ERRα inhibition notably reduced the percentage of EdU-positive cells, suggesting a decrease in DNA replication. Consistent with the in vitro assays, our in vivo tumor growth assay showed that ERRα overexpression increased PC growth whereas ERRα silencing exerted the opposite effect. Furthermore, flow cytometry analysis indicated that the suppressive effects of ERRα knockdown on the proliferation of PC may be due to induction of cell apoptosis and cell cycle arrest. Apoptosis is a crucial gene-directed program that is engaged to regulate normal cellular growth and development [28]. The apoptosis signaling cascade can be divided into 2 major pathways: the death receptor pathway (also known as extrinsic pathway) and the mitochondrial pathway (also known as intrinsic pathway) [29]. In the intrinsic pathway, cytochrome c is induced by apoptotic stimuli and released from mitochondria into cytosol, interacts with Apaf-1 and activates downstream effector caspases including caspase 3 [29,30]. Bcl-2 proteins function as gatekeepers for cytochrome c and caspase 3 acts as a vital executioner to cleave the downstream cellular substrates, such as PARP [31,32]. In our study, it was found that ERRα knockdown upregulated protein expression of cytochrome c, cleaved-caspase 3, cleaved-PARP while downregulated that of bcl-2, suggesting that intrinsic pathway was involved in ERRα knockdown-induced cell apoptosis. Aberrant function of cell cycle regulators lead to deregulation of cell cycle progression that result in uncontrolled cell proliferation. The cell cycle analysis demonstrated that ERRα knockdown significantly decreased the proportion of S phase cells and increased the G0/G1 phase cells. Consistent with the cell cycle analysis, western blot results showed that expression of CDK2, CDK4, cyclin D1 was downregulated while p21 Waf1/Cip1 and p27 Kip1 was upregulated after ERRα knockdown, confirming the cell cycle arrest at G0/G1 phase. These data validate a crucial role for ERRα in PC growth.
Pancreatic cancer is extremely aggressive and has a high metastatic potential [33]. Increasing evidence supports the notion that EMT is a vital step in the progression and metastasis of pancreatic cancer [34]. Our present study showed that silencing of ERRα notably inhibited the migration and invasion of PC cells while overexpression of ERRα enhanced that of PC cells. More important, the best characterized alteration of EMT process, such as loss of E-cadherin as well as upregulation of N-cadherin, vimentin and snail was enhanced by ERRα overexpression while suppressed by ERRα knockdown. These data suggested the oncogenic effects of ERRα on PC cell migration and invasion was associated with the activation of EMT process, indicating the potential benefits of anti-ERRα therapy for PC patients with established metastases.
Plasminogen activator inhibitor 1 (PAI1) belongs to the serpin superfamily and is a unique type of serine protease inhibitor [19]. The deregulation of PAI1 expression has been involved in various types of cancers [35], however, the specific functions and roles of PAI depend on the type of cancer. PAI1 overexpression increased cell migration and invasion in rectal cancer and triple-breast cancer [36,37], whereas it inhibits proliferation in prostate cancer [38]. In this work, we found that PAI1 is significantly overexpressed in PC tissues compare with normal tissues, and its high expression leads to shorter OS and DFS in PC patients. Subsequent in vitro assays demonstrated that knockdown of PAI1 could attenuate cell proliferation, migration and invasion enhanced by ERRα overexpression. Mechanically studies indicated that ERRα can bind to the promoter region of PAI1 and then increase its transcription.
To further explore the potential molecular mechanism by which ERRα/PAI1 promoted PC proliferation, migration and invasion, we focused our attention on the MEK/ERK pathway which had been reported to play a vital role in PC progression [24,25,39]. The western blot analysis showed that phosphorylation levels of MEK1/2 and ERK1/2 were decreased by ERRα inhibition, and knockdown of PAI1 could reverse the increasing levels of p-MEK1/2 and p-ERK1/2 induced by ERRα overexpression. Co-IP experiments were further performed and showed that PAI1 and ERK1/2 could directly interact with each other, which implied that ERRα might activated MEK/ERK in a PAI1-dependent manner. In the subsequent rescue experiments, it was noted that ERK inhibitor GDC-0994 and ERK1/2 siRNA could suppresses the cell proliferation, migration and invasion induced by overexpression of ERRα, supporting the hypothesis that ERRα/PAI1 promotes PC progression through MEK/ERK signaling pathway.
In conclusion, we determined that ERRα is significantly upregulated in PC tissues and results in poor prognosis of PC patients. ERRα can promote cell proliferation, migration and invasion via activating MEK/ERK pathway by promoting the transcription of PAI1. Our study provides the first evidence that ERRα might be a potential diagnostic and therapeutic target for PC.
Acknowledgements
This work was funded by the National Natural Science Foundation of China (No. 81672404 and No. 81974371).
Disclosure of conflict of interest
None.
Abbreviations
- PC
pancreatic cancer
- ERRα
Estrogen-related receptor alpha
- EMT
epithelial-mesenchymal transition
- PAI1
plasminogen activator inhibitor 1
- IHC
immunohistochemistry
- DMSO
dimethylsulfoxide
- qRT-PCR
quantitative real-time PCR
- MOI
multiplicity of infection
- IF
immunofluorescence
- CCK-8
Cell Counting Kit-8
- EdU
5-Ethynyl-2’-deoxyuridine
- PI
propidium iodide
- ChIP
chromatin immunoprecipitation
- qPCR
quantitative PCR
- Co-IP
Co-immunoprecipitation
- SD
standard deviations
- OS
overall survival
- DFS
disease free survival
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Garcia G, Odaimi M. Systemic combination chemotherapy in elderly pancreatic cancer: a review. J Gastrointest Cancer. 2017;48:121–128. doi: 10.1007/s12029-017-9930-0. [DOI] [PubMed] [Google Scholar]
- 3.Ohkuma R, Yada E, Ishikawa S, Komura D, Ishizaki H, Tamada K, Kubota Y, Hamada K, Ishida H, Hirasawa Y, Ariizumi H, Satoh E, Shida M, Watanabe M, Onoue R, Ando K, Tsurutani J, Yoshimura K, Yokobori T, Sasada T, Aoki T, Murakami M, Norose T, Ohike N, Takimoto M, Izumizaki M, Kobayashi S, Tsunoda T, Wada S. High expression of olfactomedin-4 is correlated with chemoresistance and poor prognosis in pancreatic cancer. PLoS One. 2020;15:e0226707. doi: 10.1371/journal.pone.0226707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tempero M. Active systemic treatment of pancreatic cancer. J Natl Compr Canc Netw. 2017;15:723–725. doi: 10.6004/jnccn.2017.0084. [DOI] [PubMed] [Google Scholar]
- 5.Jin H, Liu P, Wu Y, Meng X, Wu M, Han J, Tan X. Exosomal zinc transporter ZIP4 promotes cancer growth and is a novel diagnostic biomarker for pancreatic cancer. Cancer Sci. 2018;109:2946–2956. doi: 10.1111/cas.13737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uccello M, Moschetta M, Mak G, Alam T, Henriquez CM, Arkenau HT. Towards an optimal treatment algorithm for metastatic pancreatic ductal adenocarcinoma (PDA) Curr Oncol. 2018;25:e90–e94. doi: 10.3747/co.25.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L, Sanagapalli S, Stoita A. Challenges in diagnosis of pancreatic cancer. World J Gastroenterol. 2018;24:2047–2060. doi: 10.3748/wjg.v24.i19.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alatise OI, Ndububa DA, Ojo OS, Agbakwuru EA, Adekanle O, Arowolo OA. Pancreatic cancer in Nigeria: past, present and future. Nigerian Journal of Gastroenterology & Hepatology. 2012;1 [Google Scholar]
- 9.Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.May FE. Novel drugs that target the estrogen-related receptor alpha: their therapeutic potential in breast cancer. Cancer Manag Res. 2014;6:225–252. doi: 10.2147/CMAR.S35024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welboren WJ, Sweep FC, Span PN, Stunnenberg HG. Genomic actions of estrogen receptor alpha: what are the targets and how are they regulated? Endocr Relat Cancer. 2009;16:1073–1089. doi: 10.1677/ERC-09-0086. [DOI] [PubMed] [Google Scholar]
- 12.Chang CY, Mcdonnell DP. Molecular pathways: the metabolic regulator estrogen-related receptor α as a therapeutic target in cancer. Clin Cancer Res. 2012;18:6089–95. doi: 10.1158/1078-0432.CCR-11-3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L, Yang M, Guo X, Yang Z, Liu S, Ji Y, Jin H. Estrogen-related receptor-α promotes gallbladder cancer development by enhancing the transcription of Nectin-4. Cancer Sci. 2020;111:1514–1527. doi: 10.1111/cas.14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu Z, Ma T, Zhou J, Gao W, Li Y, Yu S, Wang Y, Chan FL. Nuclear receptor ERRα contributes to castration-resistant growth of prostate cancer via its regulation of intratumoral androgen biosynthesis. Theranostics. 2020;10:4201–4216. doi: 10.7150/thno.35589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujimura T, Takahashi S, Urano T, Kumagai J, Ogushi T, Horie-Inoue K, Ouchi Y, Kitamura T, Muramatsu M, Inoue S. Increased expression of estrogen-related receptor alpha (ERRalpha) is a negative prognostic predictor in human prostate cancer. Int J Cancer. 2007;120:2325–2330. doi: 10.1002/ijc.22363. [DOI] [PubMed] [Google Scholar]
- 16.Ye X, Guo J, Zhang H, Meng Q, Ma Y, Lin R, Yi X, Lu H, Bai X, Cheng J. The enhanced expression of estrogen-related receptor α in human bladder cancer tissues and the effects of estrogen-related receptor α knockdown on bladder cancer cells. J Cell Biochem. 2019;120:13841–13852. doi: 10.1002/jcb.28657. [DOI] [PubMed] [Google Scholar]
- 17.Liu S, Li F, Pan L, Yang Z, Shu Y, Lv W, Dong P, Gong W. BRD4 inhibitor and histone deacetylase inhibitor synergistically inhibit the proliferation of gallbladder cancer in vitro and in vivo. Cancer Sci. 2019;110:2493–2506. doi: 10.1111/cas.14102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pastushenko I, Blanpain C. EMT transition states during tumor progression and metastasis. Trends Cell Biol. 2019;29:212–226. doi: 10.1016/j.tcb.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Humphries BA, Buschhaus JM, Chen YC, Haley HR, Qyli T, Chiang B, Shen N, Rajendran S, Cutter A, Cheng YH, Chen YT, Cong J, Spinosa PC, Yoon E, Luker KE, Luker GD. Plasminogen activator Inhibitor 1 (PAI1) promotes actin cytoskeleton reorganization and glycolytic metabolism in triple-negative breast cancer. Mol Cancer Res. 2019;17:1142–1154. doi: 10.1158/1541-7786.MCR-18-0836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang JD, Ma L, Zhu Z. SERPINE1 as a cancer-promoting gene in gastric adenocarcinoma: facilitates tumour cell proliferation, migration, and invasion by regulating EMT. J Chemother. 2019;31:408–418. doi: 10.1080/1120009X.2019.1687996. [DOI] [PubMed] [Google Scholar]
- 21.Arroyo-Solera I, Pavón M, León X, López M, Gallardo A, Céspedes MV, Casanova I, Pallarès V, López-Pousa A, Mangues MA, Barnadas A, Quer M, Mangues R. Effect of serpinE1 overexpression on the primary tumor and lymph node, and lung metastases in head and neck squamous cell carcinoma. Head Neck. 2019;41:429–439. doi: 10.1002/hed.25437. [DOI] [PubMed] [Google Scholar]
- 22.Zhang LD, Chen L, Zhang M, Qi HJ, Chen L, Chen HF, Zhong MK, Shi XJ, Li QY. Downregulation of ERRα inhibits angiogenesis in human umbilical vein endothelial cells through regulating VEGF production and PI3K/Akt/STAT3 signaling pathway. Eur J Pharmacol. 2015;769:167–176. doi: 10.1016/j.ejphar.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 23.Ranhotra HS. Estrogen-related receptor alpha and mitochondria: tale of the titans. J Recept Signal Transduct Res. 2015;35:386–390. doi: 10.3109/10799893.2014.959592. [DOI] [PubMed] [Google Scholar]
- 24.Su CC. Tanshinone IIA can inhibit MiaPaCa-2 human pancreatic cancer cells by dual blockade of the Ras/Raf/MEK/ERK and PI3K/AKT/mTOR pathways. Oncol Rep. 2018;40:3102–3111. doi: 10.3892/or.2018.6670. [DOI] [PubMed] [Google Scholar]
- 25.Kang YW, Lee JE, Jung KH, Son MK, Shin SM, Kim SJ, Fang Z, Yan HH, Park JH, Han B, Cheon MJ, Woo MG, Lim JH, Kim YS, Hong SS. KRAS targeting antibody synergizes anti-cancer activity of gemcitabine against pancreatic cancer. Cancer Lett. 2018;438:174–186. doi: 10.1016/j.canlet.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 26.Vargas G, Bouchet M, Bouazza L, Reboul P, Boyault C, Gervais M, Kan C, Benetollo C, Brevet M, Croset M, Mazel M, Cayrefourcq L, Geraci S, Vacher S, Pantano F, Filipits M, Driouch K, Bieche I, Gnant M, Jacot W, Aubin JE, Duterque-Coquillaud M, Alix-Panabières C, Clézardin P, Bonnelye E. ERRα promotes breast cancer cell dissemination to bone by increasing RANK expression in primary breast tumors. Oncogene. 2019;38:950–964. doi: 10.1038/s41388-018-0579-3. [DOI] [PubMed] [Google Scholar]
- 27.Zhou S, Xia H, Xu H, Tang Q, Nie Y, Gong QY, Bi F. ERRα suppression enhances the cytotoxicity of the MEK inhibitor trametinib against colon cancer cells. J Exp Clin Cancer Res. 2018;37:218. doi: 10.1186/s13046-018-0862-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malami I, Abdul AB. Involvement of the uridine cytidine kinase 2 enzyme in cancer cell death: a molecular crosstalk between the enzyme and cellular apoptosis induction. Biomed Pharmacother. 2019;109:1506–1510. doi: 10.1016/j.biopha.2018.10.200. [DOI] [PubMed] [Google Scholar]
- 29.Matthews GM, Newbold A, Johnstone RW. Intrinsic and extrinsic apoptotic pathway signaling as determinants of histone deacetylase inhibitor antitumor activity. Adv Cancer Res. 2012;116:165–197. doi: 10.1016/B978-0-12-394387-3.00005-7. [DOI] [PubMed] [Google Scholar]
- 30.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 31.Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 32.Bao R, Shu Y, Wu X, Weng H, Ding Q, Cao Y, Li M, Mu J, Wu W, Ding Q, Tan Z, Liu T, Jiang L, Hu Y, Gu J, Liu Y. Oridonin induces apoptosis and cell cycle arrest of gallbladder cancer cells via the mitochondrial pathway. BMC Cancer. 2014;14:217. doi: 10.1186/1471-2407-14-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ayres Pereira M, Chio IIC. Metastasis in pancreatic ductal adenocarcinoma: current standing and methodologies. Genes (Basel) 2019;11:6. doi: 10.3390/genes11010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beuran M, Negoi I, Paun S, Ion AD, Bleotu C, Negoi RI, Hostiuc S. The epithelial to mesenchymal transition in pancreatic cancer: a systematic review. Pancreatology. 2015;15:217–225. doi: 10.1016/j.pan.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 35.Dellas C, Loskutoff DJ. Historical analysis of PAI-1 from its discovery to its potential role in cell motility and disease. Thromb Haemost. 2005;93:631–640. doi: 10.1160/TH05-01-0033. [DOI] [PubMed] [Google Scholar]
- 36.Muñoz-Galván S, Rivero M, Peinado-Serrano J, Martinez-Pérez J, Fernández-Fernández MC, Ortiz MJ, García-Heredia JM, Carnero A. PAI1 is a marker of bad prognosis in rectal cancer but predicts a better response to treatment with PIM inhibitor AZD1208. Cells. 2020;9:1071. doi: 10.3390/cells9051071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang W, Xu J, Fang H, Tang L, Chen W, Sun Q, Zhang Q, Yang F, Sun Z, Cao L, Wang Y, Guan X. Endothelial cells promote triple-negative breast cancer cell metastasis via PAI-1 and CCL5 signaling. FASEB J. 2018;32:276–288. doi: 10.1096/fj.201700237RR. [DOI] [PubMed] [Google Scholar]
- 38.Chen SC, Henry DO, Reczek PR, Wong MK. Plasminogen activator inhibitor-1 inhibits prostate tumor growth through endothelial apoptosis. Mol Cancer Ther. 2008;7:1227–1236. doi: 10.1158/1535-7163.MCT-08-0051. [DOI] [PubMed] [Google Scholar]
- 39.Qi ZH, Xu HX, Zhang SR, Xu JZ, Li S, Gao HL, Jin W, Wang WQ, Wu CT, Ni QX, Yu XJ, Liu L. RIPK4/PEBP1 axis promotes pancreatic cancer cell migration and invasion by activating RAF1/MEK/ERK signaling. Int J Oncol. 2018;52:1105–1116. doi: 10.3892/ijo.2018.4269. [DOI] [PMC free article] [PubMed] [Google Scholar]