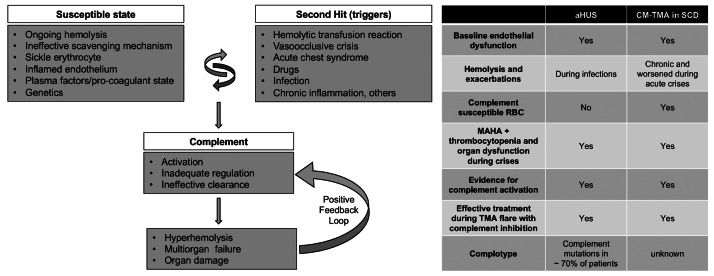
Figure 2.
A model for complement- mediated thrombotic microangiopathy in sickle cell disease. Increased understanding of complement-mediated conditions such as atypical hemolytic uremic syndrome (aHUS) and paroxysmal nocturnal hemoglobinuria has renewed interest in understanding the specific role of complement in hyperhemolysis and innate immunity. Sickle cell disease (SCD) is a prototypical disease of chronic hemolysis, where increased levels of free plasma hemoglobin and heme with an insufficient, and thus ineffective scavenging mechanism by haptoglobin and hemopexin, leads to a state that is primed for complement activation. In addition, sickle erythrocytes themselves appear to be uniquely susceptible to complement-induced hemolysis, thereby further amplifying complement activation and additional hemolysis. Like in aHUS, the already inflamed endothelium in patients with SCD can be further modulated from increased hemolysis, complement activation, coagulation dysfunction, and other plasma proteins. Further genetic studies focusing on complotype are needed to help understand its role in SCD, as they can modulate the homeostatic balance of complement activity. Triggers such as pain crisis, acute chest syndrome, infection, etc. can drive this vulnerable state very quickly into a complement activated state, which, if unregulated, can result in common and terminal complement pathway activation that can lead to catastrophic damage in end organs and even death. Irrespective of the instigating cause, once complement-mediated hyperhemolysis is set off, it could result in a positive feedback loop causing further complement activation and a precipitous drop in hemoglobin and risk of sudden death. The table on the right parallels the resting and enabling state seen in SCD to patients with aHUS. CM-TMA: complement-mediated thrombotic microangiopathy; MAHA: microangiopathic hemolytic anemia; RBC: red blood cell.