The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was discovered in Wuhan in December 2019 where it quickly led to a severe outbreak. In just a few weeks it evolved into a pandemic, with >3 million confirmed cases and >220,000 deaths attributed to COVID-19. Around 15% of infected people develop severe symptoms requiring hospitalization, and 3-10% of patients subsequently succumb to COVID-19, often due to acute respiratory distress syndrome (ARDS).1–3 Currently, neither specific treatment for COVID-19 nor a vaccine against SARS-CoV-2 is available, while trials evaluating pharmacological interventions are ongoing and specific risk groups are being defined. Whether cancer in general and hematological malignancies in particular bear substantial risks, is of eminent interest, as these patients receive immune-modulatory treatment. Of additional concern is the risk for severe COVID-19 disease in hematological malignancies given cytotoxic chemotherapy, including novel agents such as immunomodulatory drugs (IMiD) and immunotherapies (i.e., monoclonal antibodies such as daratumumab, elotuzumab and others). 2,3 It is also unclear, whether the immunosuppressant state of cancer patients predisposes them to a severe COVID-19 disease or, if a diminished host immune response may decrease the risk of multiorgan complications. Early reports from China and others indicate that cancer patients with COVID-19 may have a higher risk of a severe disease course and less favorable outcome compared to non-cancer patients, albeit not shared by others and if diligently compared with non-cancer cohorts.4–10 Most of these studies are, as yet, hampered by sample sizes, and patient cohorts consist mainly of patients with solid tumors.2–7 Other risk factors associated with adverse outcome seem to be nearly the same as for non-cancerpatients: advanced age, male sex, presence of substantial comorbidities (neurological: advanced Alzheimer, Parkinson, cardiovascular disease, diabetes mellitus, respiratory disease, chronic kidney disease, liver disease and immunosuppression).11,12
In this German Multiple Myeloma (MM) Study Group Consortium (DSMM and GMMG), we aimed to characterize a population of MM patients registered from 10 institutions who developed COVID-19 at hotspot areas in Germany. All MM patients with concomitant SARSCoV- 2 infection were treated at secondary and tertiary Comprehensive Cancer Centers (CCC). Our goal was to determine whether COVID-19 in MM patients resulted in a greater morbidity and mortality compared to prior COVID-19 reports in cancer and specifically in MM patients.
We performed a retrospective multicenter DSMM/GMMG cohort study involving 10 secondary and tertiary CCC in German pandemic epicenters. From March 1 to May 31, 2020, all MM in- and outpatients with concomitant SARS-CoV-2 infection were included (registered via DSMM/GMMG-performed incentive). SARS-CoV-2 infection was confirmed by reverse-transcriptase- PCR (RT-PCR) assay (Table 1A-B). Laboratory findings and radiological data were retrieved from the electronic medical records of each center. Comparative analysis of prior reports in cancer and MM patients was additionally performed as summarized in Table 2. The study was conducted in accordance and in compliance with the Declaration of Helsinki and International Conference on Harmonization Guidelines for Good Clinical Practice. The data cutoff date was May 31, 2020. Descriptive data with median and ranges are presented.
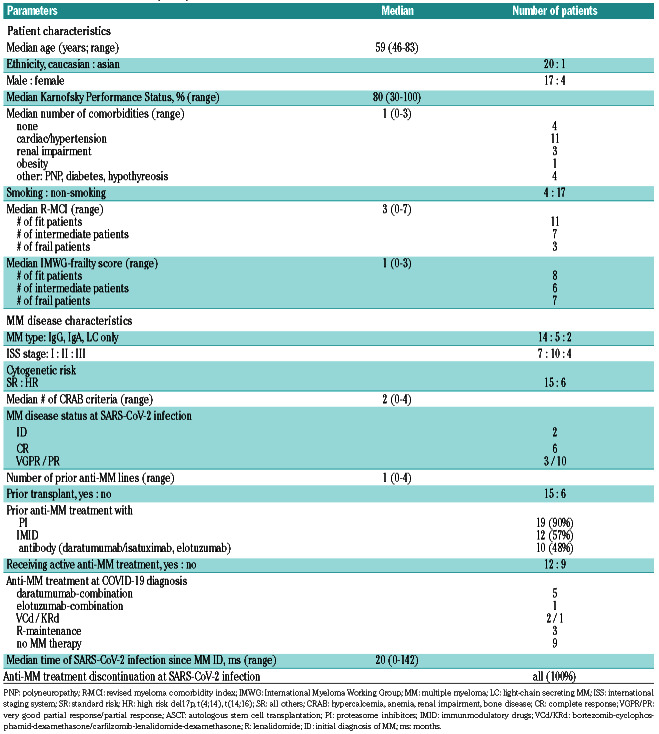
Our cohort of 21 MM patients encompassed 81% males and a median age of 59 years (range: 46-83). Most were Caucasians (Table 1A-B). Their median Karnofsky Performance Status (KPS) was 80% and the median number of comorbidities was 1 (0-3), the most prevalent being cardiovascular (hypertension), renal impairment and others (polyneuropathy [PNP], diabetes; Table 1A). Smokers versus non-smokers comprised 4 (19%) versus 17 (81%) of patients, respectively.
In line with the patients' KPS and comorbidities, the median revised myeloma comorbidity index (R-MCI)13,14 and International Myeloma Working Group (IMWG)- frailty scores14 were 3 (=fit) and 1 (=intermediate-fit), respectively. Nonetheless, 48% (via R-MCI) and 62% (via IMWG-frailty score) were in the intermediate-fit/frail group (Table 1A).
The median time from MM diagnosis to SARS-CoV-2 infection was 20 months (0-142). MM patients had a median of 1 (0-4) prior line of therapy. Fifteen (71%) patients had a prior autologous stem cell transplantation (ASCT). Preceding anti-myeloma treatments were proteasome inhibitors (PI) in 19 of 21 (90%; in all except both patients with newly diagnosed [IDMM]), IMiD in 12 of 21 (57%) and antibodies (daratumumab, elotuzumab, isatuximab) in 10 of 21 (48%) patients (Table 1A).
The most common MM subtype was IgG (67%), followed by IgA (24%) and light-chain (LC)-only MM in 9% of the patients. High-risk cytogenetics (del17p, t(4;14), t(14;16)) were present in six (29%) patients. Seven (33%), 19 (48%) and four (19%) patients had an international staging system (ISS) of 1, 2 and 3, respectively. A median of two CRAB criteria had led to anti-myeloma treatment, in line with prior reports in MM without SARS-CoV-2 infection.15
The disease status at the time of SARS-CoV-2 infection included six patients in complete remission (CR), three in very good partial remission (VGPR), 10 in partial remission (PR) and two patients with IDMM. At the time of SARS-CoV-2 infection, 12 (57%) were being treated, either with daratumumab (5), elotuzumab (1), VCd (2), KRd (1) or lenalidomide-maintenance (3). Nine patients were not on active anti-MM therapy. At the time of SARS-CoV-2 infection, anti-MM treatment was transiently stopped for ~4 weeks in all of them (Table 1A).
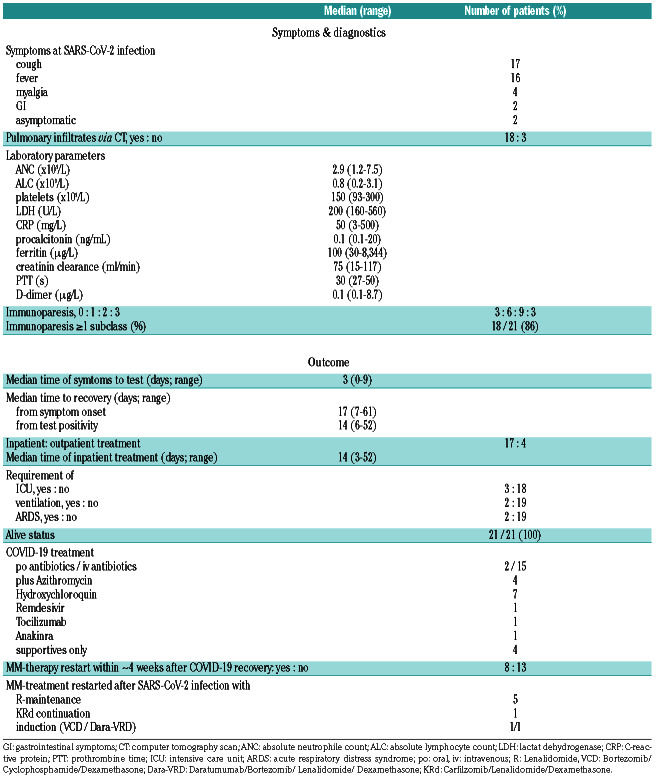
The most common reported symptoms among all patients were a cough (81%) and fever (76%). Notably, two patients were almost asymptomatic. Seventeen (81%) patients were admitted to the hospital for inpatient care. The median time between self-reported symptom onset and admission was 3 days. Three patients required intensive care unit (ICU) support, all were treated with high-flow oxygen, and two were eventually intubated. These two developed ARDS, with one already fully recovered. The median time to recovery in all patients from symptom onset was 17 days and from test positivity 14 days. There were no deaths in the total cohort.
Pulmonary infiltrates via computer tomography (CT) scans were present in 18 (86%) patients. Blood counts at SARS-CoV-2 infection showed absolute neutrophil counts (ANC) of 2.9x109/L, whereas median absolute lymphocyte counts (ALC) were suppressed (0.8x109/L). On initial presentation, platelets, lactate dehydrogenase (LDH), creatinine, PTT and D-dimer were normal or less compromised, whereas C-reactive protein (CRP) and ferritin were elevated (Table 1B). The number of patients with 1, 2 or all 3 paraprotein subclass suppression (immunoparesis) was substantial with six, nine and three patients, thus immunoparesis of >1 subclass was broadly present in 86% of patients.
Our COVID-19 management involved most frequently intravenous (iv) antibiotics in 15 patients and orally in two patients. Additionally, four patients received azithromycin and seven patients hydroxychloroquine. Remdesivir, tocilizumab and anakinra were given in one patient each who had a more severe and/or prolonged SARS-CoV-2 infection. In four patients with mild SARSCoV- 2 infection, only supportive care was required. The median time of inpatient treatment was 14 days (range: 3-52).
Approximately 4 weeks after full COVID-19 recovery, anti-MM treatment was restarted in eight patients: five patients with lenalidomide, one patient with KRd continuation with relapsed/refractory MM (RRMM), two IDMM patients received VCD and Dara-VRd induction after full SARS-CoV-2 recovery and were confirmed as SARS-CoV-2 negative by RT-PCR.
We also performed a descriptive subgroup analysis of patients in need (n=3) versus not in need (n=18) of ICU support (Online Supplementary Table S1), which revealed, that the former patients were older (60 vs. 58 years), had more comorbidities (2 vs.1; KPS: 60% vs. 90%, R-MCI: 6 vs. 3, IMWG-frailty score: 2 vs. 1), had a longer time span from symptom onset to testing (5 vs. 2 days), lower ALC counts (0.5 vs. 0.9x109/L), highly elevated inflammatory parameters and needed substantially longer to recover (from symptom onset: 55 vs. 16 days; from COVID-19- test: 52 vs. 14 days, respectively). Even though the ICUgroup predictably needed more intensified SARS-CoV-2 treatment support, the outcome in both groups was identical with 100% survival (OS; Online Supplementary Table S1).
Table 1A.
Patient characteristics (n=21).
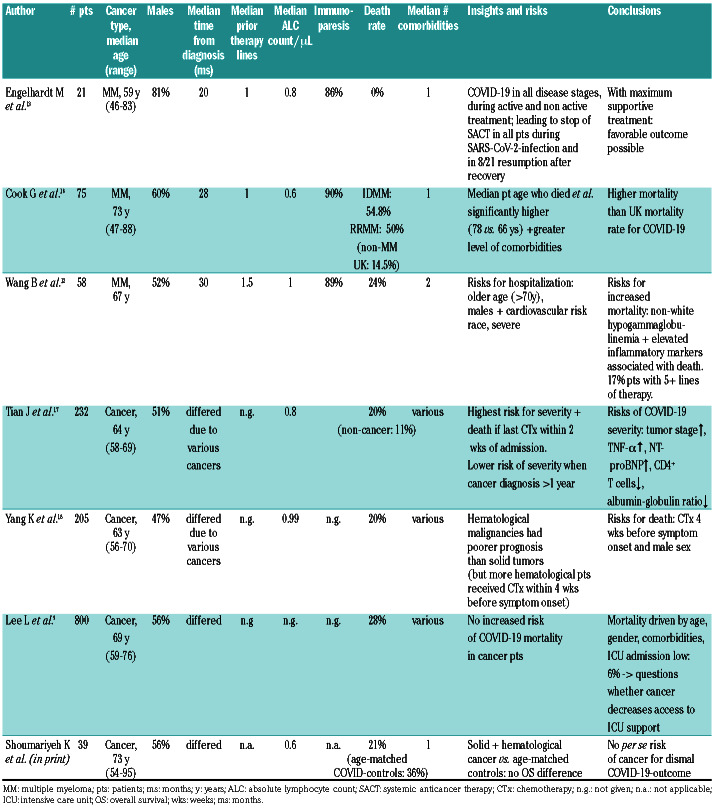
Table 2 summarizes seven selected MM/cancer-studies with SARS-CoV-2 infection. Remarkable from our data was the death rate of 0%, whilst MM disease features and characteristics were similar to those of real-life UKand US-MM-cohorts with different health systems.12,16 Even though the median patient age in both UK- and UScohorts was higher than ours and both had more nonwhites than typical in Germany, lines of prior therapy, ASCT-rates, median number of comorbidities and ALC/immunoparesis frequencies were comparable, suggesting that with maximum triage support and supportive care, MM/cancer patients per se may not have a dismal SARS-CoV-2 infection outcome.2,3,8–10 Early data in cancer patients in general5,17,18 have suggested higher risks in those with systemic/chemotherapeutic treatment within 4 weeks prior to symptom onset, male gender, and poor constitution, whereas our experience in 39 cancer patients with SARS-CoV-2 infection compared with very well matched non-cancer controls suggests no difference in OS for both groups (K. Shoumariyeh et al., Cancer Medicine 2020; in print). This is in line with a prospective observational UK study of 800 cancer patients9 that was unable to identify evidence that cancer patients on cytotoxic chemotherapy or other anticancer treatment are at an increased risk of mortality from COVID-19 disease compared with those not on active treatment. Mortality from COVID-19 in these cancer patients appeared to be principally driven by age, sex and comorbidities, as well as by cancer patients' access to intensive care support/ ICU.9
Table 1B.
COVID-19 disease characteristics (n=21).
In conclusion, we here present the examined clinical and laboratory parameters of 21 MM patients who were treated in 10 CCC with confirmed SARS-CoV-2 infection. The features of these patients were comparable to others, except that our patients were younger.12,16 We have previously described that younger MM patients may come to German tertiary centers, even though "stage and age migration" is occurring,19,20 since elderly and frail patients have recognized the advantages of university centers'/CCC' support offers. Moreover, since all regional German hospitals have been enthusiastically dealing with COVID-19 patients, we were not the only centers, where COVID-19 patients were treated, which may be different to other more centralized medical systems.
In all three MM analyses (Table 2),12,16 patient and MM disease characteristics were similar: notable was our male predominance (81%, Cook: 60%,16 Wang 52%12), the median prior lines of anti-MM treatment were in all 1, ALC and immunoparesis were present in substantial percentages and median comorbidities were 1 or 2.12,16 Nevertheless country-specific differences in death rates varied from 0% in ours to 54.8% in IDMM and 50% in RRMM in the UK cohort (non-MM UK mortality: 14.5%) and 24% in the US-cohort.12,16
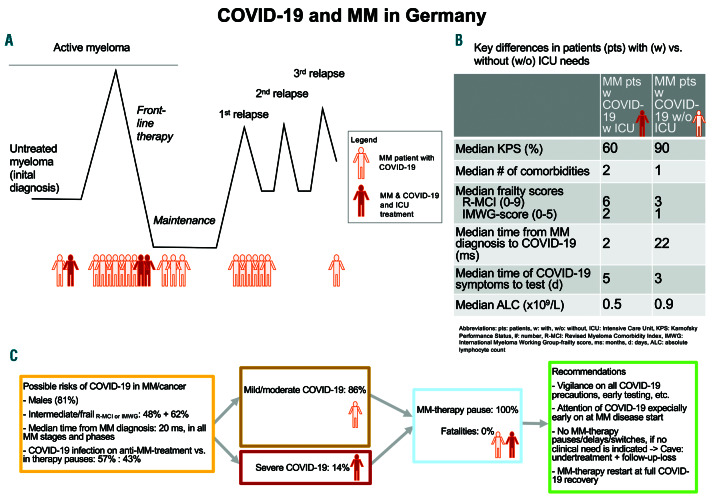
Figure 1.
Time point, when multiple myeloma (MM) patients aquired COVID-19 infection, key differences of patients with (w) versus without (w/o) intensive care (ICU) needs and learning experience of COVID-19 infection in MM. (A) Time point, when multiple myeloma (MM) patients acquired COVID-19 during their disease course, which was rather early than later and at a median of 20 months after the initial diagnosis (ID). (B) Key differences of patients w versus w/o ICU needs, who expectedly showed a worse Karnofsky performance status (KPS) in those ICU patients and who also had more comorbidities, higher R-MCI and IMWG-scores. These ICU patients also seemed to show a slightly longer time span from symptoms to COVID-19-testing and a lower absolute lymphocyte count (ALC). (C) Learning experience of possible risks for COVID-19 infection is displayed, who were in our series males in 81%, intermediate-fit or frail via R-MCI and IMWG scores in 48% and 62%, respectively and acquired COVID-19 infection fairly early during the MM course. This might be due to a "loss" of more vulnerable patients earlier (first 2 years after ID), so that later in the MM disease course, only more stoic or inured patients may survive who may be less prone to COVID-19 infection. Moreover, COVID-19 infection occurred in both patients on active MM treatment and in those without. We performed a MM therapy pause in all patients, both in milder or more substantial cases and observed no death. We recommend to stay vigilant on COVID-19, observe all precautions as described,2,3 avoid undertreatment and follow-up losses, restart anti-MM therapy with full COVID-19 recovery and encourage our MM patients and experts, that with maximum support, COVID-19 may not lead to a more dismal outcome compared to the general population.9,10
Compared to non-cancer cohorts,4,9,21,22 MM patients seemed to show a longer duration to clinical improvement (time from onset of symptoms to recovery) and longer hospitalization time of 17 and 14 days, respectively, even though intensive supportive care led to recovery. Therefore MM patients did not show a more dismal OS compared to non-cancer patients,2,3,8 which needs to be confirmed in larger analyses, with a cohort study from five New York academic centers already confirming this.10 In line, other centers have reported that MM patients have done remarkably well overall and that the type of chemotherapy being administered just prior to COVID-19 had no bearing on the outcome (personal communication: Dr. Sundar Jagannath, New York, International Myeloma Working Group [IMWG] and9,10). Although one has to be very careful with worldwide comparisons, one strategy in Germany had been to allocate all possible resources to medical centers during the COVID-19 pandemic, so that COVID-19 patients had utmost medical support, including sufficient intensive care facilities and no onrush of patients. Thus pandemicinduced overwhelming of capacities was effectively avoided, whereas if this cannot be ensured, mortality is likely to be higher (personal communication S.Jagannath and IMWG).9,10 Relevant learning experience is summarized in Figure 1.
Table 2.
Different COVID-19 outcome reports in multiple myeloma and other cancer patients.
We are well aware, that our observations have to be interpreted with caution, because our experience is as yet limited to 10 CCC in Germany. We refrained from larger subgroup analysis, because all our patients survived and we had only 3 of 21 and 2 of 21 patients who required ICU support, ventilation/intubation or had ARDS, respectively. Nevertheless, our observation of a very reassuring outcome seems to be highly encouraging, as we are aiming to continue utmost cancer care and avoid "undertreatment", appointment stops and delay of follow- ups. Our results seem also soothing to those, who are greatly threatened by SARS-CoV-2 infections and who believe that cancer patients may fare far worse, whereas an opposite observation - as shown here - may be true and is worth to be spread to our patients and colleagues (Figure 1).
Supplementary Material
Acknowledgments
ME, KS and RW specifically thank Prof. Dr. Winfried Kern (Division of Infectious Diseases, University of Freiburg (UKF)) and Prof. Dr. Hartmut Hengel (Institute of Virology, UKF) for their outstanding knowledge, support and cooperation in management of the UKF SARS-CoV-2 infected patients. ME was redeployed to general and acute medicine roles to support the COVID-19 admissions and in charge of a UKF COVID-19- ward. We thank our outstanding study coordinating and study nurse teams at all CCC for their exceptional support, great care and diligent documentation of study patients, including those with COVID-19 infection and to all our colleagues for the team spirit during the COVID-19 challenge, its pressure and during vulnerable times at pandemic hotspot areas of Germany. We also thank the UK, US and worldwide MM and cancer colleagues, including GMMG/DSMM members greatly, who we discussed our data with. Moreover, we are greatly indebted to Prof. Dr. Justus Duyster's prodigious support and the UKF-support in SARS-CoV-2 times in general, with specific gratitude to Prof. Dr. Frederik Wenz, Prof. Dr. Norbert Südkamp and Prof. Dr. Christoph Peters (all UKF).
References
- 1.Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the chinese Center for disease control and prevention. JAMA. 2020;323(13):1239-1242. [DOI] [PubMed] [Google Scholar]
- 2.Terpos E, Engelhardt M, Cook G, et al. Management of patients with multiple myeloma in the era of COVID-19 pandemic: a consensus paper from the European Myeloma Network (EMN). Leukemia. 2020;34(8):2000-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mian H, Grant SJ, Engelhardt M, et al. Caring for older adults with multiple myeloma during the COVID-19 pandemic: perspective from the International Forum for optimizing care of older adults with Myeloma. J Geriatr Oncol. 2020;11(5):764-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020; 21(3):335-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang L, Zhu F, Xie L, et al. Clinical characteristics of COVID-19- infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020;31(7):894-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai M, Liu D, Liu M, et al. Patients with cancer appear more Vulnerable to SARS-CoV-2: a Multicenter Study during the COVID- 19 outbreak. Cancer Discov. 2020;10(6):783-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Omarini C, Maur M, Luppi G, et al. Cancer treatment during the coronavirus disease 2019 pandemic: Do not postpone, do it! Eur J Cancer. 2020;133:29-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee LYW, Cazier JB, Starkey T, Turnbull CD, Kerr R, Middleton G. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395(10241):1919-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hultcrantz M, Richter J, Rosenbaum C, et al. COVID-19 infections and outcomes in patients with multiple myeloma in New York City: a cohort study from five academic centers. medRxiv. 2020. Jun 11. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Lilienfeld-Toal M, Vehreschild JJ, Cornely O, et al. Frequently asked questions regarding SARS-CoV-2 in cancer patients-recommendations for clinicians caring for patients with malignant diseases. Leukemia. 2020;34(6):1487-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang B, Van Oekelen O, Mouhieddine TH, et al. A tertiary center experience of multiple myeloma patients with COVID-19: lessons learned and the path forward. J Hematol Oncol. 2020;13(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engelhardt M, Domm A-S, Dold SM, et al. A concise revised Myeloma Comorbidity Index as a valid prognostic instrument in a large cohort of 801 multiple myeloma patients. Haematologica 2017;102(5):910-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dold SM, Möller M-D, Ihorst G, et al. Validation of the revised myeloma comorbidity index and other comorbidity scores in a multicenter German study group multiple myeloma trial. Haematologica. 2020. May 15. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gengenbach L, Reinhardt H, Ihorst G, et al. Navigating the changing multiple myeloma treatment landscape: clinical practice patterns of MM patients treated in- and outside German DSMM study group trials. Leuk Lymphoma. 2018;59(11):2692-2699. [DOI] [PubMed] [Google Scholar]
- 16.Cook G, Ashcroft AJ, Pratt G, et al. Real-world assessment of the clinical impact of symptomatic infection with severe acute respiratory syndrome coronavirus (COVID-19 disease) in patients with Multiple Myeloma receiving systemic anti-cancer therapy. Br J Haematol. 2020. May 21. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian J, Yuan X, Xiao J, et al. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21(7):893-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang K, Sheng Y, Huang C, et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21(7):904-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hieke S, Kleber M, König C, Engelhardt M, Schumacher M. Conditional survival: a useful concept to provide information on how prognosis evolves over time. Clin Cancer Res. 2015; 21(7):1530-1536. [DOI] [PubMed] [Google Scholar]
- 20.Schinke M, Ihorst G, Duyster J, Wäsch R, Schumacher M, Engelhardt M. Risk of disease recurrence and survival in patients with multiple myeloma: a German Study Group analysis using a conditional survival approach with long-term follow-up of 815 patients. Cancer. 2020;126(15):3504-3515. [DOI] [PubMed] [Google Scholar]
- 21.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu H, Stratton CW, Tang Y-W. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. J Med Virol. 2020;92(4):401-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.