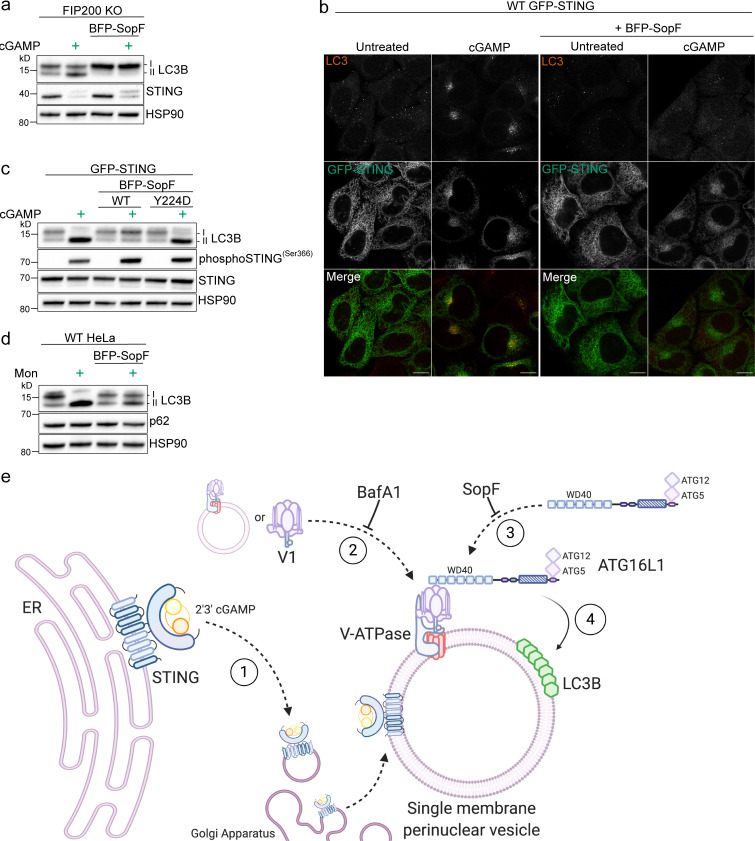
Figure 5.
The V-ATPase targeting bacterial effector, SopF, blocks STING-mediated LC3B lipidation and perinuclear foci formation. (a) FIP200 KO HeLa cells and FIP200 KO HeLa cells with stable expression of mammalian codon optimized BFP-SopF were incubated with 15 µg/ml cGAMP for 8 h. (b) Representative Airyscan confocal imaging of WT HeLa cells with stable expression of GFP-STING alone and with GFP-STING and BFP-SopF were treated with 60 µg/ml cGAMP for 8 h, fixed, and immunostained for endogenous LC3. Scale bar, 10 µm. (c) WT HeLa cells with stable expression of GFP-STING alone and with either stable expression of BFP-SopF or BFP-SopF-Y224D were incubated with 60 µg/ml cGAMP for 8 h. (d) WT HeLa cells and WT HeLa cells with stable expression of BFP-SopF were incubated with 100 μM monensin for 1 h. Western blotting experiments were independently replicated three times. (e) Model of VAIL onto single-membrane perinuclear vesicles induced by cGAMP activation of STING created with BioRender.com. (1) cGAMP-activated STING translocates from the ER to the Golgi apparatus to colocalize at or around single-membrane perinuclear vesicles. (2) The V1 complex docks to resident V0 domains in perinuclear vesicles, or vesicles with assembled V-ATPases redistribute to a denser formation in the perinuclear region. This process is blocked by BafA1. (3) ATG16L1 is recruited to interact with the V-ATPase via its WD40 domain (as reported by Xu et al., 2019). (4) LC3B is conjugated to phosphatidylethanolamine on single-membrane perinuclear vesicles by the ATG16L1-ATG5-12 complex. This process is inhibited by BafA1 and SopF. Mon, monensin.