Abstract
Psoriatic arthritis (PsA) is a heterogeneous multifaceted inflammatory artropathy, associated or not with psoriasis, part of the spondyloarthropaties group. Beyond articular and skin manifestations, patients with psoriatic disease are prone to associated comorbidities, including cardiovascular disease (CVD), obesity and metabolic syndrome, diabetes, or fatty liver disease; in order to improve the prognosis and the quality of life for these patients, it is mandatory to prevent, identify and properly manage any of the comorbidities. We aimed to assess the presence of traditional CV risk factors and MetS in a group of PsA patients, compared to controls and their possible inter-relation. We performed an observational study on 41 consecutive patients diagnosed with PsA based on CASPAR established criteria. Our subjects met the criteria of MetS in a percentage of 43.90% of the cases and AHT, frequently reported in higher percentages for PsA or psoriasis patients, compared to general population was also revealed in significant percentages by our data. Regarding dyslipidemia, it is confirmed and validated by several studies that patients diagnosed with PsA or psoriasis associate an altered lipid metabolism and our study noticed data accordingly. As PsA is a condition characterized by chronic inflammation, a non-traditional CV risk factor, each patient should benefit from a periodic close evaluation in order to approach a compete and early therapeutic intervention and reduce further CV morbidity and mortality rates.
Keywords: Psoriatic arthritis, metabolic syndrome, cardio-vascular risk factors
Introduction
Psoriatic arthritis (PsA) is a heterogeneous multifaceted inflammatory artropathy, associated or not with psoriasis, part of the spondyloarthropaties group, with both spinal and peripheral joint involvement with specific features such as enthesitis and dactylitis, as well as extra-articular immune induced involvement, like inflammatory bowel disease or ophthalmic manifestations [1,2,3].
According to related data from literature, beyond articular and skin manifestations, patients with psoriasis are prone to several comorbidities, including cardiovascular disease (CVD), obesity, metabolic syndrome, diabetes, or fatty liver disease; in order to improve the prognosis and the quality of life for these patients, it is mandatory to prevent, identify and properly manage any of the comorbidities [2,3].
CVD is a commonly seen comorbidity in rheumatic diseases, including PsA, where the systemic inflammation leads to subsequent atherosclerosis and possible major cardiovascular events, as stroke of myocardial infarction [2,3,4].
As revealed by several recent publications, between PsA patients there is an increased prevalence of arterial hypertension (AHT), diabetes, obesity and dyslipidemia, and therefore of metabolic syndrome (MetS), which exerts several auto-immune and inflammatory events similar to PsA, and consequently atherosclerotic CVD [3,4,5].
Therefore, assessing such important cardiovascular (CV) risk factors in PsA patients is clearly necessary, ideally early in the course of the disease, in order to identify them and apply proper therapeutic measures and avoid future damage and altered quality of life [5,6,7,8,9,10].
Aim
We aimed to assess the presence of traditional CV risk factors and MetS in a group of PsA patients, compared to controls and their possible inter-relation.
Material and Method
We performed an observational study on 41 consecutive patients diagnosed with PsA in Rheumatology Department of the Emergency County Hospital Craiova, based on CASPAR established criteria [6], in a one year interval between 2019-2020, and a control group including 30 subjects, with similar demographic characteristics, without inflammatory immune-mediated diseases.
The study was performed in accordance with the ethics and deonthology principles of the Helsinki Human Right’s Declaration and the study was approved by the local Ethics Committee.
All patients provided their written informed consent, after receiving a standard form which mentioned that the results would be used for research purposes.
Patients data were obtained from every patient according to the study protocol and included demographic, clinical, laboratory parameters and imagistic methods.
The presence of MetS was assessed according to the National Cholesterol Education Program (NCP) Adult Treatment Panel (ATP) III by the presence of three or more of the following conditions: reduced serum concentrations of high density lipoprotein cholesterol (HDL-C) (<40mg/dl in men and <50mg/dl in women); increased triglycerides levels (TG≥150mg/dl); hypertension (systolic/diastolic blood pressure ≥130/85mmHg); impaired glucose tolerance (fasting blood glucose levels≥100mg/dl) and abdominal obesity(waist circumference (WC) >102cm in men and >88cm in women) [7].
For statistical analysis we used GraphPad Prism 5.5 and the results are presented as mean±SD; in order to compare groups we used t-test and One-way ANOVA, and for evaluating correlations Pearson/Spearman’s coefficient.
A level of p<0.05 was considered statistically significant.
Results
Our cohort included 41 patients diagnosed with PsA, according to CASPAR criteria, and 30 controls.
For patients, we registered a mean age of 53.44±0.91 years and a mean disease duration of 6.63±4.26 years, most of them represented by women (27, 65.85%).
Evaluation of inflammatory markers showed a mean value of 11.66±26.6mg/dl for CRP and 33.27±25.59mm/h for ESR, significantly different compared to controls (3.26±12.5mg/dl for CRP and 23±27.34mm/h for ESR, p<0.001 for both values).
Regarding therapeutic management, synthetic DMARD was an option for all the patients and a combination of synthetic and biologic DMARD for 16 patients.
For disease activity, we calculated a mean DAPSA of 11.80±4.91 (min.2, max. 25.8; for PASI, the values varied between 0 and 28, with a mean of 15.32±7.12.
The general data of the patients are presented in Table 1.
Table 1.
General data of the study group
|
Patients (N) |
41 |
|
Female (N; %) |
27 (65.86) |
|
Male (N; %) |
14 (34.14) |
|
Age (years) |
54.34±0.91 |
|
Disease duration (years) |
6.63±4.26 |
|
Type of psoriasis (N; %) Nail Skin Nail and skin Sine psoriasis |
24 (58.53) 35 (85.36) 24 (58.53) 5 (12.19) |
|
Type of psoriatic arthritis (N; %) Peripheral Axial and peripheral |
32 (78.04) 9 (21.95) |
|
CRP (mg/dl) |
11.66±26.60 |
|
ESR (mm/h) |
32.27±25.59 |
|
DAPSA |
11.80±4.91 |
|
PASI |
15.32±7.12 |
|
BMI (kg/m2) |
27.44±6.35 |
|
Uric acid (mg/dl) |
4.77±1.48 |
|
Current medication DMARD non biologic (N; %) DMARD biologic (N; %) |
41 (100) 16 (39.02) |
|
AHT (N;%) |
21 (53.65) |
|
Diabetes (N;%) |
8 (19.52) |
|
Smoking (N;%) |
20 (48.78) |
|
Dyslipidemia (N;%) |
28 (68.29) |
CRP-C reactive protein; ESR-erythrocyte sedimentation rate; DAPSA-Disease Activity in PSoriatic Arthritis; PASI-Psoriatic Area and Severity Index
Assessing the presence of MetS, we reckoned 18 patients that met at least 3 of the 5 criteria established for diagnosis.
When analyzing the presence altered lipid parameters, we found dyslipidemia for 28 of the patients and 10 controls (OR 2.43, 95% CI 0.95-6.22) (Figure 1).
Figure 1.
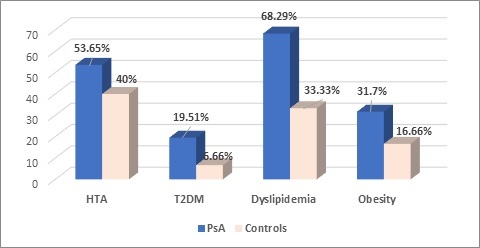
Comparison of clinical characteristics for PsA/controls
Regarding each of the lipids parameters, we found total cholesterol (TC) in levels above the limits in 28 of the 41 patients (68.29%), compared to 11 (36.66%) of the controls, p=0.002.
The mean value for TC was different between the two groups (208.4±40.91 for APS, 186.3±31.88 for controls, p=0.015).
HDL cholesterol fraction (HDL-C) was also different between the two groups (60.96±15.24mg/dl for patients and 68.14±15.24mg/dl for controls, p=0.04), the same observation being noticed also for LDL cholesterol fraction (LDL-C), 108.7±121.5mg/dl vs. 88.48±102.8mg/dl, p=0.005. TC-HDL ratio had a mean value of 3.71+0.734, 12 patients having values over 3.5.
Hypertriglyceridemia was observed for a statistically significant, higher percentage of the patients, compared to controls (25; 60.97% versus 7; 16.33%, p=0.015), (Table 2, Figure 2).
Table 2.
Clinical and laboratory parameters PsA patients/controls
|
|
PsA |
Controls |
p |
||
|
|
Mean |
SD |
Mean |
SD |
|
|
Age (years) |
54.34 |
0.91 |
48.21 |
12.2 |
0.740 |
|
BMI (kg/m2) |
27.44 |
6.35 |
25.4 |
3.9 |
0.361 |
|
SBP (mmHg) |
126.2 |
23.43 |
121.2 |
35.3 |
0.272 |
|
DBP (mHg) |
77.47 |
17.65 |
69.8 |
27.5 |
0.367 |
|
Blood glucose (mg/dl) |
94 |
13 |
82 |
11 |
0.221 |
|
TC (mg/dl) |
208.4 |
40.91 |
186.3 |
31.88 |
0.002 |
|
LDL-C (mg/dl) |
115.1 |
20.27 |
95.64 |
15.72 |
0.005 |
|
HDL-C (mg/dl) |
60.96 |
15.24 |
68.14 |
22.71 |
0.04 |
|
TC/HDL C ratio |
3.71 |
0.73 |
3.21 |
0.93 |
0.64 |
|
TG (mg/dl) |
125.6 |
104.8 |
121.3 |
53.2 |
0.66 |
|
Waist circumference (cm) |
93.88 |
14.3 |
92.07 |
14.28 |
0.81 |
Figure 2.

Bar plot (mean with SEM) of the lipid parameters for PsA/controls
For PsA patients, we registered a mean BMI of 27.44±6.35, with 11 (26.82%) overweight patients and 13 (31.70%) obese, compared to 6 (20%) overweight patients and 5 (16.66%) with a BMI over 30 of the control group (Figure 1).
Blood glucose tended to be higher in the patients group, but without being statistically significant (p=0.221), with a mean value of 94±13mg/dl for PsA and 82±11mg/dl for controls. Diabetes was an established diagnosis for 8 of the patients (19.52%), compared to 6.66% of the control group.
Uric acid had in our study, a mean value relatively similar for the two categories studied (4.77±1.48mg/dl PsA/4.11±1.02mg/dl controls).
Smoking was identified in 20 of the patients and 12 controls.
The diagnosis of hypertension (AHT) was known for 21 of the PsA patients (53.65%), all of them receiving treatment according to cardiologists’ indications, significantly different compared to controls (12; 40%), p=0.09.
At our evaluation, we reckoned a mean SBP of 126.2±23.43mmHg and a mean DBP of 77.47±17.65mmHg.
We considered that analyzing the possible inter-relation between AHT and other cardiovascular risk factors is benefic, taking under consideration that all the patients had controlled values of BP, that could somehow influence our results.
There was a positive, moderate correlation between the presence AHT and LDL-C (r=0.359, p=0.02), TG (r=0.50, p<0.001) and a moderate negative correlation between AHT and HDL-C (r=-0.385, p=0.031).
No significant inter-relation was observed between AHT and blood glucose.
Discussion
Metabolic syndrome, a world-wide health problem, implies a multi-organic pro-inflammatory state, associating several well-established CV risk factors.
Our subjects met the criteria of MetS in a percentage of 43.90% of the cases.
The data reported are variable, a study published by Raychaundri et al, found an increased percentage of PsA patients associating MetS (58.1%) [8], as well as a study that included 109 patients, published by Mok et al, with a percentage of 38% [9], while Souza et al, in 2019, found a percentage of 50% of the PsA patients with MetS [10].
PsA is a chronic, inflammatory disease, associated with several extra-articular features and comorbidities which imply a systemic pro-inflammatory status, endothelial dysfunction and subclinical atherosclerosis, even from early stages, when signs and symptoms are not clinical obvious [11].
Cardiovascular disease (CVD) is more prevalent between patients with chronic inflammatory musculoskeletal diseases compared to their healthy counterparts [12].
AHT is frequently reported in higher percentages for PsA or psoriasis patients, compared to general population [12,13], observation revealed also by our data.
The reported prevalence varies between studies [13], our analysis revealing a percentage of 53.65%.
A recent meta-analysis, that included over 17000 PsA patients reported similar significantly increased risk of a high blood pressure (both systolic and diastolic) [14].
Diabetes and insulin resistance are often linked to rheumatic diseases, as well as to a higher CV risk. The data regarding diabetes, type 2, suggest a significant risk among patients with PsA, rheumatoid arthritis (RA) or ankylosing spondylitis (AS) [9,15,16,17].
Possible due to a relative low number of patients, our results revealed a percentage of 19.51% patients with diabetes, however increased compared to controls (6.66%).
Obesity was observed in 31.70% of the patients and overweight in 11 (26.82%) of them, prevalence reported by a recent publication, in 2019, by Quiero R et al, which included 290 consecutive PsA subjects [18].
This data is, nevertheless, sustained by previous scientific reports [9,11,19,20].
It is of outmost importance not to neglect the input of adipose tissue and obesity which are reported to be sources for proinflammatory cytokines, directly related to systemic inflammation and to an increased, progressive atherosclerosis, even subclinical if not evaluated or at the beginning of the disease. Moreover, it seems that there is a link between obesity and IL-7 mediated disease, including PsA [18].
Regarding dyslipidemia, it is confirmed and validated by several studies that patients diagnosed with PsA or psoriasis associate an altered lipid metabolism and our study noticed data accordingly. The most commonly reported abnormalities are represented by increased values of TC, LDL-C and TG. Furthermore, we reckoned lower levels of HDL-C, a traditional CV risk factor, similar to several other publications [21,22,23,24].
Conclusions
As PsA is a condition characterized by chronic inflammation, a non-traditional CV risk factor, each patient should benefit from a periodic close evaluation, in order to approach a compete and early therapeutic intervention and reduce further CV morbidity and mortality rates.
Conflict of interests
None to declare.
Acknowledgments
Acknowledgments
Beatrice Andreea Chisălău and Cristina Dorina Pârvănescu share equal contributions to this work.
References
- 1.Özlem Ö, Ayten Y, Aysun SA, Duygu TK, Özlem OI, Senem T. Are there any differences among psoriasis, psoriatic arthritis and rheumatoid arthritis in terms of metabolic syndrome and cardiovascular risk factors. Eur J Rheumatol. 2019;6(4):174–178. doi: 10.5152/eurjrheum.2019.19029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haddad A, Zisman D. Comorbidities in Patients with Psoriatic Arthritis. Rambam Maimonides Med J. 2017;8(1):e0004–e0004. doi: 10.5041/RMMJ.10279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Puig L. Cardiometabolic Comorbidities in Psoriasis and Psoriatic Arthritis. Int J Mol Sci. 2018;19(1):E58–E58. doi: 10.3390/ijms19010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feld J, Nissan S, Eder L, Rahat MA, Elias M, Rimar D, Laor A, Bitterman H, Zisman D. Increased Prevalence of Metabolic Syndrome and Adipocytokine Levels in a Psoriatic Arthritis Cohort. J Clin Rheumatol. 2018;24(6):302–307. doi: 10.1097/RHU.0000000000000721. [DOI] [PubMed] [Google Scholar]
- 5.Caso F, Del Puente A, Oliviero F, Peluso R, Girolimetto N, Bottiglieri P, Foglia F, Benigno C, Tasso M, Punzi L, Scarpa R, Costa L. Metabolic syndrome in psoriatic arthritis: the interplay with cutaneous involvement. Evidences from literature and a recent cross-sectional study. Clin Rheumatol. 2018;37(3):579–586. doi: 10.1007/s10067-017-3975-0. [DOI] [PubMed] [Google Scholar]
- 6.Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 2006;54(8):2665–2673. doi: 10.1002/art.21972. [DOI] [PubMed] [Google Scholar]
- 7.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 8.Raychaudhuri SK, Chatterjee S, Nguyen C, Kaur M, Jialal I, Raychaudhuri SP. Increased prevalence of the metabolic syndrome in patients with psoriatic arthritis. Metab Syndr Relat Disord. 2010;8(4):331–334. doi: 10.1089/met.2009.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mok CC, Ko GT, Ho LY, Yu KL, Chan PT, To CH. Prevalence of atherosclerotic risk factors and the metabolic syndrome in patients with chronic inflammatory arthritis. Arthritis Care Res (Hoboken) 2011;63(2):195–202. doi: 10.1002/acr.20363. [DOI] [PubMed] [Google Scholar]
- 10.Souza CS, de Castro CCS, Carneiro FRO, Pinto JMN, Fabricio LHZ, Azulay-Abulafia L, Romiti R, Cestari TF, Suzuki CE, Biegun PM, Guedes LS, Oyafuso LKM. Metabolic syndrome and psoriatic arthritis among patients with psoriasis vulgaris: Quality of life and prevalence. J Dermatol. 2019;46(1):3–10. doi: 10.1111/1346-8138.14706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peluso R, Caso F, Tasso M, Ambrosino P, Dario Di, Lupoli R, Criscuolo L, Caso P, Ursini F, Puente AD, Scarpa R. Cardiovascular Risk Markers and Major Adverse Cardiovascular Events in Psoriatic Arthritis Patients. Rev Recent Clin Trials. 2018;13(3):199–209. doi: 10.2174/1574887113666180314105511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Apraş Bilgen Ş, Kalyoncu U, Erden A, Canpolat U1, Kılıç L, Karadağ Ö, Aytemir K, Kiraz S, Akdoğan A, Ertenli İ. Assessment of subclinical atherosclerosis in psoriatic arthritis patients without clinically overt cardiovascular disease or traditional atherosclerosis risk factors. Turk Kardiyol Dern Ars. 2018;46(5):358–365. doi: 10.5543/tkda.2018.36169. [DOI] [PubMed] [Google Scholar]
- 13.Husted JA, Thavaneswaran A, Chandran V, Eder L, Rosen CF, Cook RJ, Gladman DD. Cardiovascular and other comorbidities in patients with psoriatic arthritis: a comparison with patients with psoriasis. Arthritis Care Res (Hoboken) 2011;63(12):1729–1735. doi: 10.1002/acr.20627. [DOI] [PubMed] [Google Scholar]
- 14.Choudhary S, Patel R, Pradhan D, Deval R, Singh H, Thomas G, Jain AK. Psoriasis and cardiovascular disorders: association or epiphenomenon? Meta-analysis of observational studies. Biotech. 2020;10(3):104–104. doi: 10.1007/s13205-020-2089-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han C, Robinson DW, Hackett MV, Paramore LC, Fraeman KH, Bala MV. Cardiovascular disease and risk factors in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol. 2006;33(11):2167–2172. [PubMed] [Google Scholar]
- 16.Solomon DH, Love TJ, Canning C, Schneeweiss S. Risk of diabetes among patients with rheumatoid arthritis, psoriatic arthritis and psoriasis. Ann Rheum Dis. 2010;69(12):2114–2117. doi: 10.1136/ard.2009.125476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dubreuil M, Rho YH, Man A, Zhu Y, Zhang Y, Love TJ, Ogdie A, Gelfand JM, Choi HK. Diabetes incidence in psoriatic arthritis, psoriasis and rheumatoid arthritis: a UK population-based cohort study. Rheumatology (Oxford) 2014;53(2):346–352. doi: 10.1093/rheumatology/ket343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Queiro R, Lorenzo A, Tejón P, Coto P, Pardo E. Obesity in psoriatic arthritis: Comparative prevalence and assvbmociated factors. Medicine (Baltimore) 2019;98(28):e16400–e16400. doi: 10.1097/MD.0000000000016400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhole VM, Choi HK, Burns LC, Vera Kellet C, Lacaille DV, Gladman DD, Dutz JP. Differences in body mass index among individuals with PsA, psoriasis, RA and the general population. Rheumatology (Oxford) 2012;51(3):552–556. doi: 10.1093/rheumatology/ker349. [DOI] [PubMed] [Google Scholar]
- 20.Kumar S, Han J, Li T, Qureshi AA. Obesity, waist circumference, weight change and the risk of psoriasis in US women. J Eur Acad Dermatol Venereol. 2013;27(10):1293–1298. doi: 10.1111/jdv.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pietrzak A, Chabros P, Grywalska E. Serum lipid metabolism in psoriasis and psoriatic arthritis-an update. Arch Med Sci. 2019;15(2):369–375. doi: 10.5114/aoms.2018.74021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tekin NS, Tekin IO, Barut F, Sipahi EY. Accumulation of oxidized low-density lipoprotein in psoriatic skin and changes of plasma lipid levels in psoriatic patients. Mediators Inflamm. 2007;2007:78454–78454. doi: 10.1155/2007/78454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azfar RS, Gelfand JM. Psoriasis and metabolic disease: epidemiology and pathophysiology. Curr Opin Rheumatol. 2008;20(4):416–422. doi: 10.1097/BOR.0b013e3283031c99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pietrzak A, Chodorowska G, Szepietowski J, ZalewskaJanowska A, Krasowska D, Hercogová J. Psoriasis and serum lipid abnormalities. Dermatol Ther. 2010;23(2):160–173. doi: 10.1111/j.1529-8019.2010.01311.x. [DOI] [PubMed] [Google Scholar]


