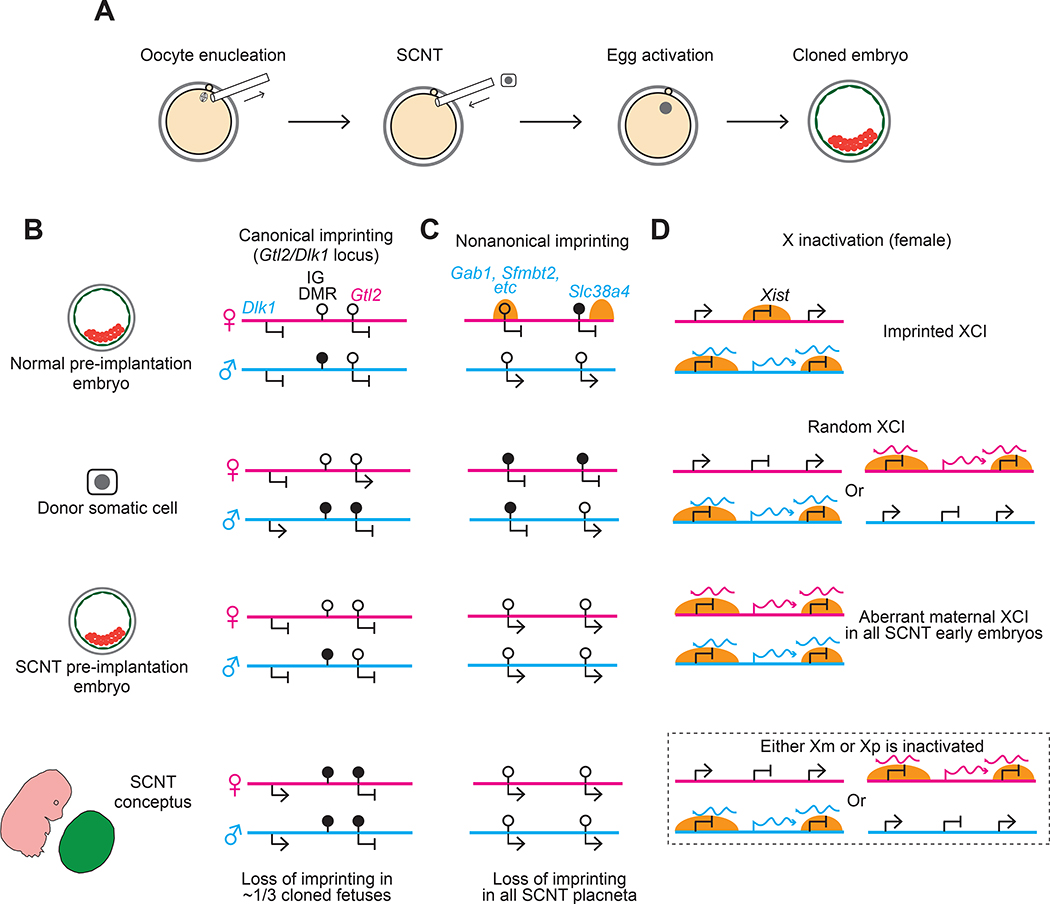
Figure 4. Defects in genomic imprinting and imprinted X inactivation occur in somatic cell nuclear transfer reprogramming.
A) Depicted are the general processes involved in somatic cell nuclear transfer (SCNT). A metaphase II oocyte is first enucleated and a donor cell nucleus from a differentiated somatic cell is transferred to the enucleated oocyte. The oocyte is then artificial activated by applying a chemical or electrical stimulus to initiate the developmental program to form cloned embryos.
B) Stochastic loss of canonical imprinting occurs at the Gtl2/Dlk1 locus in SCNT embryos. At this locus, the ICR is known as IG DMR and paternal-specific DNA methylation of the donor somatic cell is maintained in pre-implantation embryos derived by SCNT. However, after implantation, around one third of SCNT embryos exhibit gain of DNA methylation on the normally unmethylated maternal allele. The abnormal gain of DNA methylation is associated with biallelic expression of Dlk1 and biallelic repression of Gtl2, and with fetal lethality.
C) Imprinting is consistently lost at noncanonically imprinted loci in SCNT embryos. In donor cells such as cumulus and sertoli cells, the typical noncanonical imprinted loci (represented by Gab1) are marked by neither maternal H3K27me3 nor somatic differentially methylated regions (DMRs). Therefore, all placenta derived by SNCT show loss of noncanonical imprinting; they are also enlarged, which may be caused by disrupted noncanonical imprinting. Although the DMR is maintained at the noncanonically imprinted Slc38a4 locus in the donor cell, Slc38a4 becomes bi-allelically expressed in all cloned embryos, suggesting that the Slc38a4 DMR cannot be maintained without maternal H3K27me3 in early embryos.
D) Aberrant X inactivation occurs in SCNT embryos. In donor cells, one X-chromosome is randomly inactivated and Xist is not marked by maternal H3K27me3. Therefore, cloned embryos always express ectopic maternal Xist in addition to paternal Xist, and both X-chromosomes undergo XCI. Analogous to Eed maternal knockout embryos or XpXp androgenetic embryos, the bi-allelic inactivation of XCI is likely to be resolved at the late blastocyst stage (dashed boxes). However, insufficient expression of X-linked genes in pre-implantation development may still contribute to the post-implantation defects of cloned embryos, and correction of Xist expression has been shown to increase cloning efficiency by about 10-fold 122