Abstract
Hypoxia leading to stabilization of hypoxia-inducible factor 1α (HIF-1α) serves as an early upstream initiator for adipose tissue (AT) dysfunction. Monocyte-derived macrophage infiltration in AT contributes to inflammation, fibrosis and obesity-related metabolic dysfunction. It was previously reported that myeloid cell-specific deletion of Hif-1α protected against high-fat diet (HFD)-induced AT dysfunction. Prolyl hydroxylases (PHDs) are key regulators of HIF-1α. We examined the effects of myeloid cell-specific upregulation and stabilization of Hif-1α via deletion of prolyl-hydroxylase 2 (Phd2) and whether interleukin-1 receptor associated kinase-M (Irak-M), a known downstream target of Hif-1α, contributes to Hif-1α-induced AT dysfunction. Our data show that with HFD, Hif-1α and Irak-M expressions were increased in the AT macrophages of Phd2flox/flox/LysMcre mice compared with LysMcre mice. With HFD, Phd2flox/flox/LysMcre mice exhibited increased AT inflammation, fibrosis, and systemic insulin resistance compared with control mice. Furthermore, Phd2flox/flox/LysMcre mice bone marrow-derived macrophages exposed to hypoxia in vitro also had increased expressions of both Hif-1α and Irak-M. In wild-type mice, HFD induced upregulation of both HIF-1a and Irak-M in adipose tissue. Despite equivalent expression of Hif-1α compared with wild-type mice, globally-deficient Irak-M mice fed a HFD exhibited less macrophage infiltration, decreased inflammation and fibrosis and improved glucose tolerance. Global Irak-M deficiency was associated with an alternatively-activated macrophage phenotype in the AT after HFD. Together, these data show for the first time that an Irak-M-dependent mechanism likely mediates obesity-related AT dysfunction in conjunction with Hif-1α upregulation.
Keywords: hypoxia-inducible factor 1α, interleukin-1 receptor-associated kinase-M, obesity, prolyl-hydroxylase 2
INTRODUCTION
Obesity is a major public health problem worldwide that is associated with systemic inflammation, insulin resistance, type 2 diabetes mellitus, cardiovascular disease and increased all-cause mortality (11). Recent studies show that inflammation and fibrosis are hallmarks of a metabolically dysfunctional adipose tissue (AT) that is associated with insulin resistance and glucose intolerance (28, 30). Identifying proximal events responsible for AT dysfunction is important in the development of novel therapeutic strategies for obesity-related conditions.
In the face of excess calories, AT stores energy through adipocyte enlargement and recruitment of adipogenic precursor cells (28); however, an inadequate angiogenic response to the rapid adipocyte expansion results in hypoxia and stabilization of hypoxia-inducible factor 1α (HIF-1α) (29). Overexpression of a constitutively active form of Hif-1α in adipocytes results in AT dysfunction that is characterized by macrophage infiltration, inflammation and fibrosis (12). Conversely, selective genetic inhibition of Hif-1α in adipocytes results in reduced AT fibrosis and inflammation, and increased insulin sensitivity in mice (27). This literature has established the scientific premise that Hif-1α in adipocytes serves as an early upstream initiator for AT dysfunction (28, 29).
Macrophage infiltration in AT is thought to contribute to inflammation, fibrosis and obesity-related metabolic dysfunction (14). Previous work has demonstrated that in the setting of obesity, crown-like structures (CLS) formed by AT macrophages are hypoxic, resulting in classically-activated pro-inflammatory macrophage phenotype that has shown to be both HIF-1α-dependent and independent (10). Specific deletion of Hif-1α in myeloid cells in mice fed a high-fat diet (HFD) is protective against AT inflammation, CLS formation and systemic insulin resistance indicating that the HIF-1α pathway is necessary in mediating the effects of AT macrophages on AT dysfunction (33).
Prolyl hydroxylases (PHDs) are key regulators of HIF-1α (16) allowing the von Hippel-Lindau protein to bind and target HIF-1α for proteasomal degradation under conditions of normoxia (17). During hypoxia, HIF-1α is not degraded and translocates to the nucleus and binds to hypoxia response elements in DNA. Three HIF prolyl hydroxylases have been identified (PHD1, PHD2, PHD3) (5); with PHD2 being the key oxygen sensor setting the steady-state levels of HIF-1 α in normoxia (4).
It is not known whether myeloid-cell specific upregulation of Hif-1α via Phd2 deletion results in AT inflammation and dysfunction. Furthermore, the specific downstream mechanisms of Hif-1α in AT macrophages that are responsible for the metabolic dysfunction in obesity have not been identified. A recent report has shown that pharmacological activation and overexpression of the homologous HIF-1α in human monocytes increased interleukin-1 receptor associated kinase-M (IRAK-M) expression (26). These data indicate that Irak-M is a downstream target of Hif-1α in macrophages that could potentially mediate the metabolic dysfunction associated with obesity.
In the current study, we tested the hypothesis that specific deletion of Phd2 in myeloid cells results in increased Hif-1α expression that is associated with an increase in Irak-M expression, thereby contributing to AT dysfunction.
RESEARCH DESIGN AND METHODS
All experimental protocols were submitted to and approved by the Institutional Animal Care and Use Committee (IACUC) of The Ohio State University.
Generation and Maintenance of Mice
Phd2flox/flox/LysMcre.
Mice were generated by crossing a C57BL/6J mouse containing loxP sequences on either side of the Phd2 gene (Phd2flox/flox; from Dr. Guo-Hua Fong of the University of Connecticut) (32) with a C57BL/6J mouse expressing cre recombinase from the lysozyme M promoter (LysMcre), thereby yielding a homozygous mouse with PHD2-deficient monocytes and macrophages (Phd2flox/flox/LysMcre). Age- and sex-matched LysMcre mice were used as the control group. Only male mice were used in all experiments to avoid the confounding effect of the estrous cycle.
Irak-M−/−.
A colony of Irak-M−/− (globally-deficient) mice bred on a C57BL/6J background for more than 10 backcrosses was first established at the University of Michigan, Ann Arbor, Michigan (7). Age- and sex-matched C57BL/6 wild-type mice (The Jackson Laboratory, Bar Harbor, Maine) served as control. Only male mice were used in all experiments.
Mice were maintained in a temperature-controlled animal facility with a 12-h dark and 12-h light cycle and housed in groups of two to five, with unlimited access to water and normal chow, as indicated in individual experiments. All mice were maintained in a pathogen-free facility. For all the high-fat-diet (HFD) feeding experiments, mice were fed with a diet containing 60% of its calories from fat (TD.06414; Envigo) for 12 wk. HFD experiments were initiated at the age of 6–8 wk.
Insulin Tolerance Test and Oral Glucose Tolerance Test
For insulin tolerance test (ITT), mice were fasted for 6 h before administration of insulin (Humulin-R, Eli Lilly) at 1 U/kg body weight by intraperitoneal (IP) injection. Venous blood samples were collected by tail-bleeding method at indicated time points. Blood glucose levels (mg/dL) were measured using Accu-Chek glucometer (Roche). Mice were denied food throughout the experiment. For oral glucose tolerance test (OGTT), mice were fasted for 6 h before administration of glucose (2 g/kg body weight) by oral gavage. Venous blood samples were collected by tail-bleeding method at indicated time points. Blood glucose levels (mg/dL) were measured using Accu-Chek glucometer (Roche). Mice did not have access to food throughout the experiment.
Harvesting and Preparation of Tissues
After the mice were euthanized, an incision at the level of the xiphoid process was made to open the thoracic cavity, exposing the heart and lungs. The peritoneal cavity was then opened and the epididymal white adipose tissue was harvested, using care to avoid any gonadal tissues. Adipose tissues were then snap-frozen in dry ice and stored at −80°C until further analysis.
Adipocyte and Adipose Tissue Macrophage Purification
Macrophages and adipocytes from adipose tissue were isolated via collagenase digestion as described previously (23). Briefly, epididymal white adipose tissue were excised and minced in Hanks’ Balanced Salt Solution (HBSS). Minced tissues were then transferred into 10-mL round-bottom tube containing ice-cold digestion buffer (HBSS with Ca2+ and Mg2+ supplemented with 0.5% BSA). Collagenase (Sigma) was added at 1 mg/mL and suspensions were incubated at 37°C for 30 min with vigorous shaking. After collagenase digestion, 10 mM of EDTA was added and then incubated at 37°C for an additional 10 min. The cell suspension was filtered through a 100 µm nylon filter and then spun at 300 g for 5 min to separate floating adipocytes from the stromal vascular fraction (SVF) pellet. Isolation of CD11b+ cells from SVF isolates was performed by magnetic immunoaffinity isolation with anti-CD11b antibodies conjugated to magnetic beads (10 µl per 1 × 107 cells, MACS Miltenyi Biotec). Cells were isolated using positive selection columns before preparation of whole-cell lysates.
Real-Time Reverse Transcriptase Polymerase Chain Reaction (real-time RT-PCR)
Total RNA were extracted from tissues in TRIzol (Invitrogen) using a tissue lyser and then isolated using established protocols (24). The quality and quantity of the RNA were determined by absorbance at 260 and 280 nm using Epoch microplate spectrophotometer (BioTek, Winooski, VT). cDNAs were prepared by reverse transcribing 500 ng of total RNA with Multiscribe reverse transcriptase (ThermoFisher) using manufacturer’s instructions. Quantitative real-time PCRs (qPCRs) were carried out on QuantStudio3 qPCR Machine (Applied Biosystems, Foster City, CA) using Fast SYBR Green Master Mix (Applied Biosystems). The primers have been published previously (12) and were purchased from Integrated DNA Technologies (Coralville, IA). The relative amounts of all mRNAs were calculated by using the comparative threshold cycle (2–∆∆Ct) method (19) using hypoxanthine phosphoribosyl transferase (Hprt) or glyceraldehyde-3-phosphate dehydrogenase (Gapdh) mRNA as the invariant control.
Protein Analysis
Adipose tissue lysate preparation.
Harvested adipose tissue (200 mg) were homogenized in 800 µL lysis buffer (20 mM tris-HCl, 1 mM EDTA, 255 mM sucrose, protease inhibitor) using zirconia/silica beads and tissue lyser. Following homogenization, the tissues were centrifuged at 5000xg for 10 min at 4°C to remove the fat cake and tissue debris, leaving the adipose tissue lysate as the supernatant.
ELISA.
Interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α) and c-c motif chemokine ligand 2 (CCL2/JE) protein levels were measured in the adipose tissue lysate using ELISA kits (BioLegend, San Diego, California) according to manufacturer’s instructions.
Hypoxia Exposure of Bone Marrow-Derived Macrophages
Bone marrow derived macrophages (BMDMs) were isolated from Phd2flox/flox/LysMcre and control (LysMcre) mice according to standard protocols (36). Bone marrow cells were counted and seeded in a sterile petri dish. The medium with macrophage colony-stimulating factor (CSF1/M-CSF) was changed every other day. After 7 days in culture, BMDMs were plated in 100-mm culture dishes and incubated with and without CoCl2 (100µM) for 6 h or placed in custom-made (20), sealed Plexiglas chambers (10 × 11 × 20 cm) and exposed to hypoxic gas mixture hypoxia (95% N2+5% CO2) or normoxia for 30 min.
Immunoblot
BMDMs were lysed in buffer containing RIPA (Sigma) supplemented with protease inhibitors (Roche Diagnostics), protein concentration was measure by BCA protein assay. Equal amount of protein was loaded, then gels were subjected to electrophoresis. Membranes were incubated with antibodies: anti-HIF1α (Cell Signaling #14179), anti-PHD2 (Cell Signaling #4835) and anti-β Actin (Cell Signaling #3700) in 1:1000 dilution; secondary antibody was anti-rabbit (Cell Signaling #7074 and anti-mouse (Cell Signaling #7076) in 1:3000 dilution. Both antibodies for the Western blots (anti-HIF1α and anti-PHD2) have been previously validated (9, 35). Signals were developed with an ECL Plus Western blot detection kit (Amersham, Arlington Heights, IL). Densitometry was calculated using Image J.
Histology and Image Analysis
Adipose tissue was excised and fixed in neutral-buffered 10% formalin for 72 h. Following paraffin embedding, the tissue sections were stained with Masson’s trichrome using standard protocols. For immunohistochemistry (IHC), sections were deparaffinized. After antigen retrieval and blockage of endogenous peroxidase, sections were stained with primary antibodies against F4/80 (E-Bioscience, 1:200) followed by biotinylated secondary antibodies (anti-mouse and anti-rabbit antibodies), as described previously (27). Sections were mounted and visualized using Leica Microsystems (Wetzlar, Germany). For image analysis, digital images of 10 random tissue sections per animal were captured under low power (×20) objective. Stains for trichrome and F4/80 were objectively quantified using histogram analysis in Adobe Photoshop CS5 software for the appropriate pixels according to a previously published method (8). The histogram analysis was performed by an investigator blinded to experimental design.
Statistical Analysis
All results were given as means ± standard deviation (SD). For comparison of two independent groups, Mann-Whitney U test was used. Differences between the two groups over time were determined by a two-way analysis of variance (ANOVA) for repeated measures, with a subsequent Sidak’s multiple comparisons test. Statistical analyses were performed using GraphPad Prism 8.0 (GraphPad Software, La Jolla, California). Levels of significance were denoted as follows: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Significance was accepted at a p value of < 0.05.
RESULTS
Generation of Transgenic Mice with Myeloid-Specific Knockdown of Phd2
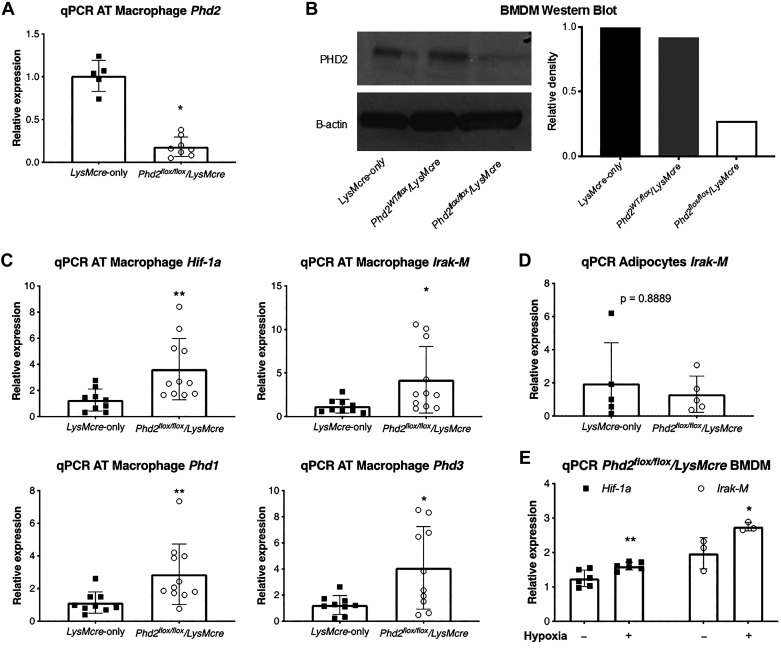
We isolated macrophages from the AT and bone marrow of Phd2flox/flox/LysMcre mice and LysMcre-only mice fed with normal chow. The reductions in Phd2 mRNA expression and PHD2 protein expression in AT macrophages and BMDM of Phd2flox/flox/LysMcre compared with LysMcre mice are shown in Fig. 1A and Fig. 1B, respectively. Figure 1, C and D show the effects of myeloid cell deletion of Phd2 in mice fed with HFD in AT macrophages and adipocytes, respectively. In AT macrophages, there was a threefold increase in Hif1a expression in Phd2flox/flox/LysMcre mice compared with LysMcre-only mice (Fig. 1C). The reduced Phd2 expression was accompanied by an increase in mRNA expression of Phd1 and Phd3, the other isoforms of Phd (Fig. 1C), consistent with previous reports (21, 22). There was increased expression of Irak-M, a purported downstream effector of HIF-1α, in AT macrophages of Phd2flox/flox/LysMcre mice (Fig. 1C). There was no difference in expression of Irak-M in isolated adipocytes (Fig. 1D) between LysMcre and Phd2flox/flox/LysMcre mice fed HFD in contrast to adipose tissue (AT) macrophages. These data demonstrate that Phd2 deletion results in elevated Hif1a and Irak-M expressions in AT macrophages with HFD. In addition, BMDMs of Phd2flox/flox/LysMcre mice (normal chow) exposed to hypoxia in vitro had increased expressions of both Hif1a and Irak-M compared with normoxia (Fig. 1E). BMDMs of Phd2flox/flox/LysMcre fed with normal chow exposed to hypoxia showed increased levels of HIF-1α protein compared with control LysMcre mice (Figure S2 in the Supplemental Data) (https://doi.org/10.6084/m9.figshare.11465595).
Fig. 1.
Phd2 inhibition in macrophages leads to overexpression of Hif-1α and its downstream effector Irak-M. A: qPCR analysis of Phd2 expression in macrophages isolated from adipose tissue (AT) of Phd2flox/flox/LysMcre and LysMcre control mice (n = 5–8 per group). B: Western blot analysis of bone marrow-derived macrophages (BMDM) of Phd2flox/flox/LysMcre and control mice (samples pooled from 2 to 3 mice per group). Right: quantitative analysis of band density by ImageJ software. C: qPCR analysis of Hif-1a in adipose tissue macrophages in mice fed with high-fat diet (HFD), together with Hif-1α downstream effector Irak-M, and two other isoforms of Hif-1α prolyl-hydroxylases Phd1 and Phd3 (n = 9–11 per group). D: qPCR analysis of Irak-M in isolated adipocytes of Phd2flox/flox/LysMcre and LysMcre control mice fed a HFD. E: qPCR analysis of BMDM of Phd2flox/flox/LysMcre mice exposed to normoxic or hypoxic gas mixture. The readings are normalized by Hprt and Gapdh. **P < 0.01, *P < 0.05.
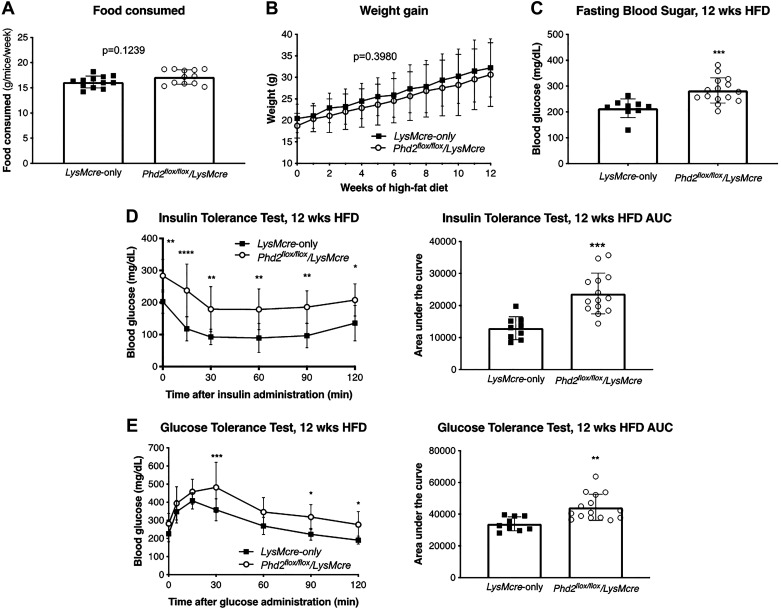
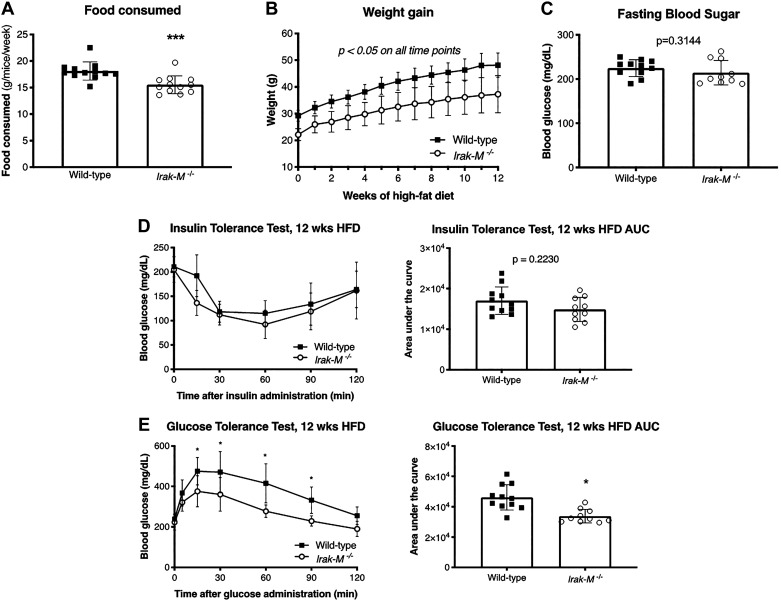
Knockdown of Phd2 in Macrophages Leads to Impaired Glucose Metabolism After 12 wk of HFD
To investigate the metabolic consequences of macrophage-specific Hif-1α overexpression by Phd2 knockdown, Phd2flox/flox/LysMcre mice and LysMcre-only controls were challenged with HFD for 12 wk. During the 12-wk HFD exposure, Phd2flox/flox/LysMcre mice consumed equal amount of food (Fig. 2A) and gained the same amount of body weight compared with control (Fig. 2B). Despite similar weight gain, Phd2flox/flox/LysMcre exhibited increased fasting blood sugar (Fig. 2C). Furthermore, Phd2flox/flox/LysMcre mice demonstrated insulin resistance, or blunted response to insulin, as judged by intraperitoneal insulin tolerance test (Fig. 2D, left). The difference is evident upon comparison of the area under the curve (AUC) of their insulin responses (Fig. 2D, right). Moreover, the OGTT demonstrated that Phd2flox/flox/LysMcre mice exhibited glucose intolerance, especially at 30 min post-oral glucose challenge (Fig. 2E, left). This is also evident upon comparison of their AUCs (Fig. 2E, right). In a separate experiment, LysMcre (n = 6) and Phd2flox/flox/LysMcre (n = 8) mice at a similar age (8 wk) to the HFD experiments were used and fed normal chow (NC) for 12 wk instead of HFD. The results are shown in Figure S1 in the Supplemental Data (https://doi.org/10.6084/m9.figshare.11465595). There were no differences in the ITT and OGTT values between the LysMcre and Phd2flox/flox/LysMcre mice after 12 wk of NC (Figure S1A and Figure S1B). Phd2flox/flox/LysMcre mice had increased insulin resistance (Figure S1A) and glucose intolerance (Figure S1B) with HFD. LysMcre mice fed a HFD tended to have higher glucose values during the ITT compared with LysMcre mice fed NC, but these did not achieve statistical significance (P = 0.26). LysMcre mice fed a HFD have higher glucose values during the OGTT compared with LysMcre mice fed NC. Overall, these results show that knockdown of Phd2 in myeloid cells exacerbates the metabolic dysfunction with HFD.
Fig. 2.
Knockdown of Phd2 in macrophages leads to impaired glucose metabolism after 12 wk of high-fat diet (HFD). A: amount of food consumed by LysMcre control and Phd2flox/flox/LysMcre mice during HFD. B: body weight gain in LysMcre and Phd2flox/flox/LysMcre mice during 12 wk of HFD (n = 9–15 per group). C: fasting glucose levels in LysMcre and Phd2flox/flox/LysMcre mice after HFD (n = 9–15 per group). D: blood glucose response curves of LysMcre and Phd2flox/flox/LysMcre during intraperitoneal insulin tolerance test after HFD, and their corresponding area under the curve (AUC) on the right (n = 9–14 per group). E: blood glucose response curves of LysMcre and Phd2flox/flox/LysMcre during oral glucose tolerance test after HFD, with the corresponding AUC on the right (n = 9–15 per group). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
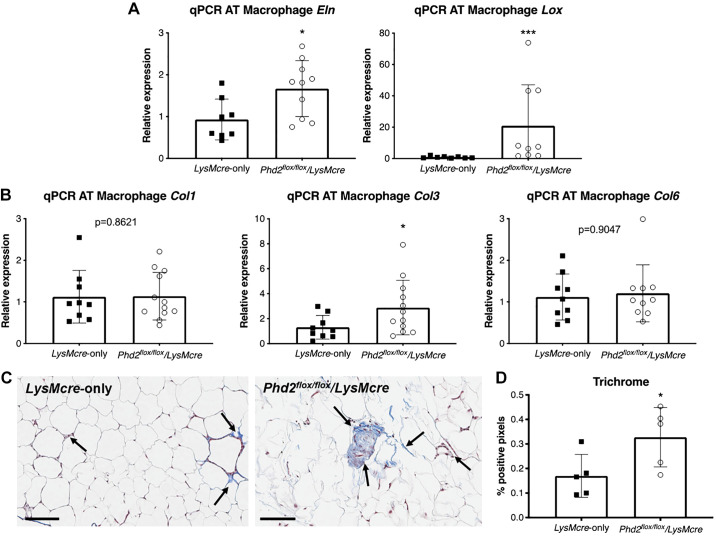
Knockdown of Phd2 in Macrophages Leads to Increased Adipose Tissue Fibrosis After 12 wk of HFD
Recent studies show that fibrosis is a hallmark of metabolically-dysfunctional adipose tissue (29). To further investigate the deleterious effects of Hif1a overexpression in macrophages of obese mice, we sought to determine whether Phd2 knockdown can exacerbate fibrosis and pathological extracellular matrix (ECM) expansion of adipose tissue during HFD exposure. qPCR shows that fibrotic markers elastin (Eln) and lysyl oxidase (Lox), a transcriptional target of Hif-1α known to cross-link collagen fibers, are upregulated in AT macrophages of Phd2flox/flox/LysMcre mice (Fig. 3A). Among the collagens, collagen III was noted to have increased expression in AT macrophages in Phd2flox/flox/LysMcre mice compared with LysMcre alone (Fig. 3B). Consistent with gene expression analysis, histological examination of the epididymal white adipose tissue with Masson’s trichrome stain (Fig. 3C) demonstrated enhanced ECM deposition in adipose tissue of obese Phd2flox/flox/LysMcre (Fig. 3D). Together, these data demonstrate that the loss of Phd2 in myeloid cells results in enhanced fibrosis as measured by elevated gene expression and increased ECM by histological evidence.
Fig. 3.
Knockdown of Phd2 in macrophages leads to increased adipose tissue fibrosis after 12 wk of HFD. A: qPCR analysis of elastin (Eln) and lysyl oxidase (Lox) in LysMcre-only control and Phd2flox/flox/LysMcre mice (n = 9–10 per group). B: qPCR analysis of collagens I, III, and VI (Col1, Col3, Col6) in LysMcre and Phd2flox/flox/LysMcre mice (n = 9–12 per group). C: Masson’s trichrome stain (arrows) for adipose tissue of LysMcre (left) and Phd2flox/flox/LysMcre transgenic mice (right). Black bars indicate 100 μm. D: quantitative analysis of positive pixels for trichrome stain (n = 5 animals per group, mean of 10 tissue sections per animal). *P < 0.05, ***P < 0.001.
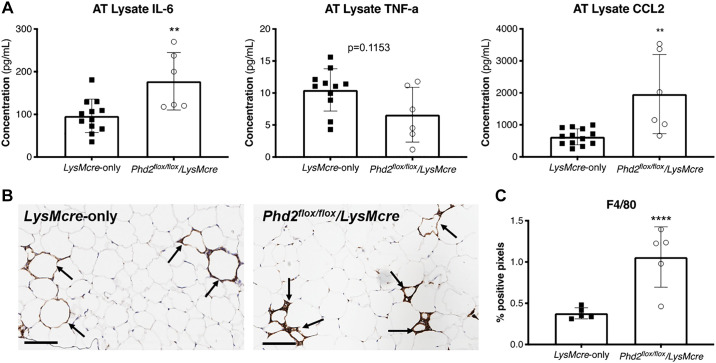
Knockdown of Phd2 in Macrophages Leads to Increased Adipose Tissue Inflammation and Macrophage Infiltration after 12 wk of HFD
To determine the role of Phd2 in regulating inflammation in adipose tissue following HFD, we measured inflammatory cytokines and the degree of macrophage infiltration in the adipose tissue in Phd2flox/flox/LysMcre mice compared with LysMcre-only mice. ELISA of the adipose tissue lysate revealed increased concentration of IL-6, and CCL2, but not TNF-α, in Phd2flox/flox/LysMcre mice compared with control (Fig. 4A). Immunohistochemical analysis of adipose tissue with antibodies against the macrophage marker F4/80 (Fig. 4B) confirmed increased macrophage infiltration in Phd2flox/flox/LysMcre (Fig. 4C). These demonstrate that Phd2 knockdown in myeloid cells regulates IL-6 production and macrophage infiltration in adipose tissue.
Fig. 4.
Knockdown of Phd2 in macrophages leads to increased adipose tissue inflammation and macrophage infiltration after 12 wk of HFD. A: ELISA of adipose tissue lysate of LysMcre-only control and Phd2flox/flox/LysMcre mice (n = 6–13 per group) for interleukin-6 (IL-6), tumor necrosis factor alpha (TNF), and c-c motif chemokine ligand 2 (CCL2). B: immunohistochemical staining of F4/80 in adipose tissue of LysMcre (left) and Phd2flox/flox/LysMcre transgenic mice (right). Arrows indicate the crown-like structures (CLS) formed by macrophage aggregation in HFD-fed mice. The bars indicate 100 μm. C: quantitative analysis of positive pixels for F4/80 stain (n = 5 animals per group, mean of 10 tissue sections per animal). **P < 0.01, ****P < 0.0001.
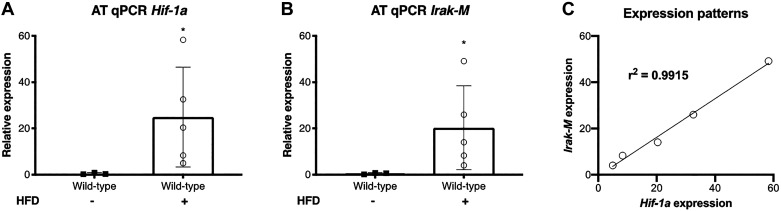
HFD Induces Expression of Hif1a and Irak-M in Adipose Tissue
We fed WT mice with either normal chow or HFD and determined the differential expressions of Hif1a and Irak-M in the adipose tissue. As expected, obesity induced upregulation of Hif1a in the adipose tissue (Fig. 5A) that was associated with an increase in Irak-M expression after HFD challenge (Fig. 5B). To compare their patterns of expression, we correlated the levels of expression of Hif1a and Irak-M (Fig. 5C). We observed parallel increase in relative expression of these two genes; that is, an increase in Hif1a expression translated to about the same magnitude of Irak-M induction (R2 = 0.99, P = 0.0003) Together, these data demonstrate that HFD exposure leads to substantial induction of Hif1a that is proportional to the level of Irak-M upregulation, suggesting that Hif1a modulates Irak-M expression in the adipose tissue of obese mice.
Fig. 5.
High-fat diet (HFD) induces Irak-M expression in adipose tissue. A: qPCR analysis of Hif1a in adipose tissue of wild-type mice fed with either normal chow or HFD (n = 3–5 per group). B: qPCR analysis of Irak-M in adipose tissue of wild-type mice fed with either normal chow or HFD (n = 3–5 per group). C: correlation of gene expression patterns of Hif1a and Irak-M in wild-type mice fed with HFD (n = 5). *P < 0.05.
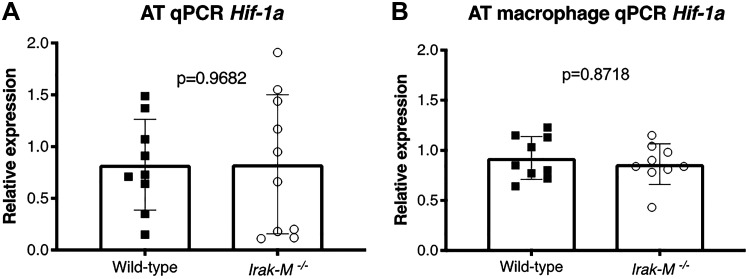
Deficiency in Irak-M Improves Glucose Tolerance after 12 wk of HFD
To determine whether Irak-M is critical in the development of obesity-induced AT dysfunction, WT and globally-deficient Irak-M−/− mice were challenged with HFD for 12 wk. Compared with WT mice the Irak-M−/− mice consumed slightly less amount of food (Fig. 6A). The WT mice had increased baseline weight compared with Irak-M−/− mice but there were no differences in the absolute weight gain between the WT mice (18.9 ± 4.7 g) and Irak-M−/− mice (15.1 ± 5.7 g) during 12 wk of HFD (P = 0.11) (Fig. 6B). Although there were no differences in fasting blood sugar levels (Fig. 6C), Irak-M−/− exhibited better glucose tolerance during the OGTT compared with WT controls (Fig. 6E), despite equivalent expressions of Hif1a in the whole adipose tissue and AT macrophages (Fig. 7). Globally-deficient Irak-M−/− mice also exhibited lower glucose values during the ITT, although these did not achieve statistical significance (repeated measures ANOVA, P = 0.105) (Fig. 6D, left). Overall, the absence of Irak-M, a known downstream effector of Hif-1α, leads to a more favorable metabolic profile with HFD. This is despite equivalent expressions of Hif1a compared with WT mice (Fig. 7).
Fig. 6.
Deficiency in Irak-M improves glucose tolerance after HFD. A: amount of food consumed of wild-type (WT) and Irak-M−/− mice during HFD. B: body weight gain in WT and Irak-M−/− mice during 12 wk of HFD (n = 10–11 per group). C: fasting glucose levels in WT and Irak-M−/− mice after HFD (n = 10–11 per group). D: blood glucose response curves of WT and Irak-M−/− mice during intraperitoneal insulin tolerance test after HFD, and their corresponding AUC on the right (n = 10–11 per group). E: blood glucose response curves of WT and Irak-M−/− mice during oral glucose tolerance test after HFD, with the corresponding AUC on the right. *P < 0.05, ***P < 0.001.
Fig. 7.
HIF-1α expression in whole adipose tissue (left) and adipose tissue macrophage (right) of wild-type and Irak-M−/− mice.
Deficiency in Irak-M Reduces Fibrosis in Adipose Tissue after 12 wk of HFD
In this study, we sought to determine whether the pathological adipose tissue fibrosis conventionally associated with HFD challenge can be suppressed by Irak-M inhibition. Consistent with our observations in lung fibrosis (3), there is substantial downregulation in the mRNA expression of collagens I, III and VI in whole adipose tissue of globally-deficient Irak-M−/− mice compared with WT after 12 wk of HFD (Fig. 8A). In line with these transcriptional changes, Masson’s trichrome stain of the adipose tissue (Fig. 8B) indicated that ECM deposition is significantly reduced in the Irak-M−/− mice (Fig. 8C).
Fig. 8.
Deficiency in Irak-M reduces fibrosis in adipose tissue after 12 wk of HFD. A: qPCR analysis of collagens I, III, and VI (Col1, Col3, Col6) in wild-type (WT) control and Irak-M−/− mice (n = 9–11 per group). B: Masson’s trichrome stain (arrows) for adipose tissue of WT control (left) and Irak-M−/− mice (right). Bars indicate 100 μm. C: quantitative analysis of positive pixels for trichrome stain (n = 8 animals per group, mean of 10 tissue sections per animal). *P < 0.05, ***P < 0.001.
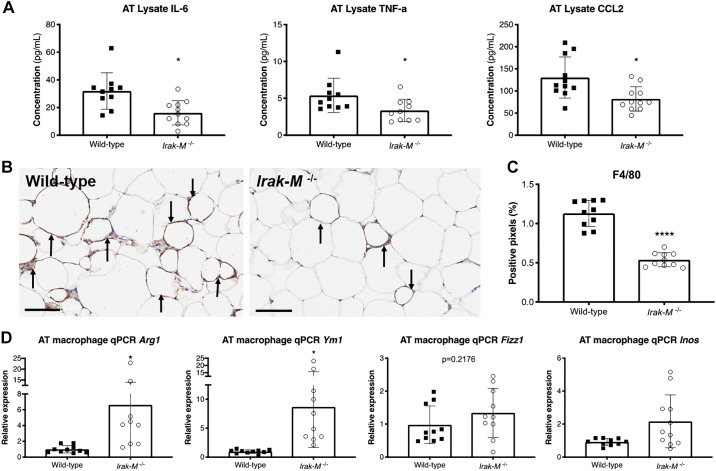
Deficiency in Irak-M Reduces AT Inflammation, Macrophage Infiltration and Skews Macrophages toward an Alternatively-Activated M2-Like Phenotype after 12 wk of HFD
After HFD challenge, adipose tissue isolated from globally- deficient Irak-M mice had decreased concentrations of IL-6, TNF-α and CCL2 when compared with WT mice (Fig. 9A). There were no significant differences (P > 0.05) in the circulating levels of these inflammatory cytokines in the plasma (Figure S3 in the Supplemental Data) (https://doi.org/10.6084/m9.figshare.11465595). In addition, we measured a decrease in the number of infiltrating macrophages in HFD-fed Irak-M−/− compared with WT mice when histological sections were stained with F4/80 (Fig. 9, B and C). Given that the local microenvironment shapes macrophage activation, we characterized the activation phenotype of macrophages in the adipose tissue by measuring the gene expression of classically- (inducible nitric oxide synthase [Inos/Nos2]) and alternatively-activated (Arg1, Ym1, Fizz1) macrophage markers by qPCR. Compared with control, there was significant upregulation of Arg1 and Ym1 in Irak-M−/− mice (Fig. 9D). These data indicate that global inhibition of the Hif-1α effector Irak-M leads to reduction of IL-6 and TNFα-mediated adipose tissue inflammation, with corresponding decrease in CCL2-mediated macrophage recruitment, and polarization of local adipose tissue macrophages toward the anti-inflammatory, alternatively- activated phenotype.
Fig. 9.
Deficiency in Irak-M reduces adipose tissue inflammation, macrophage infiltration, and skews macrophages toward an alternatively activated M2-like phenotype after 12 wk of HFD. A: ELISA of adipose tissue lysate of wild-type (WT) and Irak-M−/− mice (n = 10–11 per group) for interleukin-6 (IL-6), tumor necrosis factor α (TNF), and c-c motif chemokine ligand 2 (CCL2). B: immunohistochemical staining of F4/80 in adipose tissue of WT (left) and Irak-M−/− mice (right). Arrows indicate the crown-like structures (CLS) formed by macrophage aggregation in HFD-fed mice. The black bars indicate 100 μm. C: quantitative analysis of positive pixels for F4/80 stain (n = 10 animals per group, mean of 10 tissue sections per animal). D: qPCR analysis for M1/M2 markers (Arg1, Ym1, Fizz1, and Inos/Nos2) of macrophages isolated from adipose tissue (n = 9–11 per group). *P < 0.05, ****P < 0.0001.
DISCUSSION
We examined the role of Phd2 in regulating Hif-1α overexpression in macrophages utilizing Phd2flox/flox/LysMcre mice fed with HFD. Our data show that Hif-1α and Irak-M expressions were increased in the AT macrophages of Phd2flox/flox/LysMcre mice fed with HFD. Furthermore, these mice exhibited increased systemic insulin resistance and AT inflammation compared with control mice fed with HFD. We also showed that Phd2flox/flox/LysMcre mice BMDMs exposed to hypoxia in vitro had increased expressions of both Hif1a and Irak-M. Despite equivalent expression of Hif1a compared with WT mice, globally-deficient Irak-M mice fed with HFD exhibited greater glucose tolerance, decreased AT fibrosis, inflammation, and macrophage infiltration. Global Irak-M deficiency was associated with an alternatively activated macrophage phenotype in the AT after HFD. Together, these data demonstrated, for the first time, an Irak-M-dependent mechanism by which Hif-1α expression in macrophages mediates obesity-related AT dysfunction.
In obesity, the adipose tissue responds to caloric excess by storing triglycerides through adipocyte hypertrophy and hyperplasia. This rapid tissue expansion creates a state of hypoxia in adipose tissue, leading to the induction of the master transcription factor, Hif-1α (28). The results of our current study are congruent with prior work that Hif-1α in myeloid cells promotes AT remodeling toward insulin resistance. Takikawa and colleagues showed that myeloid cell-specific Hif-1α deletion protected against HFD-induced inflammation, macrophage infiltration, and systemic insulin resistance (33). In our study, myeloid cell-specific deletion of Phd2 resulted in Hif-1a overexpression with consequent increase in AT dysfunction and systemic insulin resistance.
Using pharmacologic and genetic approaches, previous studies have established the important role of adipocyte Hif-1α in obesity-induced adipose tissue dysfunction and insulin resistance. Overexpression of Hif-1α in adipocytes caused metabolic dysfunction, whereas adipocyte-selective inhibition of Hif-1α had the opposite beneficial effect (12, 18, 27). Existing studies on the effects of hypoxia on adipose tissue dysfunction have been studied largely in terms of adipocyte Hif-1α, with limited studies on the possible role of AT macrophage Hif-1α as well as its downstream effectors. In this current study, we demonstrated that overexpression of Hif-1a in macrophages in obese mice via deletion of Phd2 is associated with impaired glucose metabolism (Fig. 2), adipose tissue fibrosis (Fig. 3), adipose tissue inflammation and increased macrophage infiltration (Fig. 4). Similar to macrophage functions in other tissues, AT macrophages are recruited to phagocytose cellular debris following adipocyte necrosis. Adipokine signals released from these dying cells, such as CCL2 function as chemoattractant for circulating monocytes (1). Recruited macrophages surround the dead adipocytes, visualized histologically in the AT as CLS. These macrophages are, by themselves, hypoxic (10) and display profound proinflammatory features, thereby contributing to collagen deposition and AT fibrosis in mice. Consistent with this, our current work demonstrated that overexpression of Hif-1a in macrophages is associated with increased production of CCL2 in adipose tissue (Fig. 4B). Enhanced macrophage infiltration was associated with increased fibrosis of adipose tissue (Fig. 3), as well as increased production of inflammatory markers IL-6 and TNF-α (Fig. 4B). Previous work has determined that macrophages, rather than adipocytes themselves, mainly produce proinflammatory cytokines which significantly impair the insulin sensitivity of adipocytes (25, 34). These results collectively suggest the pivotal importance of macrophage Hif-1a in pathological expansion of adipose tissue.
We were interested in identifying downstream mediators of macrophage Hif-1a that could contribute to the AT dysfunction in obesity. Recently, it was shown that HIF-1a is an important regulator of IRAK-M (26) in human monocytes. Irak-M was initially thought to be exclusively expressed in myeloid cells, but recent studies have shown it to be expressed in structural cells, such as epithelial cells (6, 13, 31). Studies showed that pharmacological activation and overexpression of HIF-1α in human monocytes increased IRAK-M expression (26). Thus, Irak-M offers a downstream target of Hif-1α that could potentially mediate the metabolic dysfunction associated with obesity. Our results demonstrate that obesity in WT mice induced a robust upregulation of Irak-M consistent with increased expression of Hif1a in AT (Fig. 5). Furthermore, obese globally-deficient Irak-M mice exhibited improved glucose tolerance (Fig. 6) associated with decreased adipose tissue fibrosis (8). We have previously demonstrated that Irak-M is an important regulator in fibrotic responses, specifically in the setting of lung fibrosis. We showed that globally-deficient Irak-M mice were protected from bleomycin-induced pulmonary fibrosis, indicating that Irak-M is a key mediator of fibrogenesis in response to bleomycin (3). Our current data suggest that Irak-M also mediates adipose tissue fibrosis during pathological fat expansion in obesity.
Previous work has demonstrated that HIF-1α-induced upregulation of IRAK-M skews monocytes toward pro-inflammatory phenotype during sepsis in humans. Because Irak-M is a negative regulator of toll-like receptor (TLR) pathway, it is conceivable that its upregulation, through Hif-1α, should concomitantly inhibit pro-inflammatory response (26). Interestingly, our results showed otherwise. We noted that global Irak-M deficiency favored the anti-inflammatory alternatively activated phenotype and actually contributed to reduced inflammation of the adipose tissue. Our findings are seemingly in contrast with Hulsman et al., who reported that IRAK-M is downregulated in circulating blood monocytes in obese individuals, contributing to chronic inflammation in obesity (15). We argue that the profile of circulating blood monocytes is different from recruited macrophages in the adipose tissue. The local microenvironment in the adipose tissue shapes the phenotype of macrophages, as the macrophage response is highly contextual and dictated by the nature of the inciting event.
Our study has limitations. First, as in our prior studies (2, 3), we used control mice from a different colony in the Irak-M experiments. However, results from our current experiments suggest that the deficiency of Irak-M is the one responsible for the improvements in metabolic abnormalities rather than differences in mice strain. Upregulation of Hif-1a in adipose tissue has been shown to promote metabolic dysfunction (12). In WT mice, HFD induces upregulation of both Hif-1a and Irak-M in adipose tissue (Fig. 5, A and B). On the other hand, HFD induces equivalent upregulation of Hif-1a in adipose tissue of WT and Irak-M−/− mice (Fig. 7); these findings would not be expected if the improvements seen in Irak-M−/− are due solely to mice strain differences. Second, we used a global rather than a macrophage-specific deletion of Irak-M. As mentioned, recent studies show that Irak-M is expressed in structural cells, such as epithelial cells, and not exclusively in myeloid cells. Within adipose tissue, however, there was no difference in expression of Irak-M in isolated adipocytes (Fig. 1D) between LysMcre and Phd2flox/flox/LysMcre mice fed HFD in contrast to adipose tissue (AT) macrophages. Thus, although we used a global knockdown of Irak-M, our results likely represent effects seen due to changes in macrophages. Nonetheless, given the global nature of the Irak-M depletion, we acknowledge the possibility that tissues other than myeloid cells may also be involved in our observed results. In addition, future studies are needed to show that knockdown of Irak-M in Phd2flox/flox/LysMcre mice reverses the metabolic abnormalities. Third, we did not have Irak-M−/− and WT mice on normal chow as a control in the experiments shown in Figs. 6–9. Thus, it is difficult to know if the improvements in glucose tolerance and adipose tissue dysfunction with HFD are due to loss of Irak-M, or that the HFD was ineffective in causing weight gain and impaired metabolism in this mouse strain. Fourth, the metabolic abnormalities with HFD in Hif-1α myeloid cell overexpression may involve tissues other that AT, such as the liver and islets of Langerhans. Finally, it is possible that other proteins may also be involved other than HIF-1α stabilization causing the metabolic abnormalities after Phd2 deletion. Further studies will be needed to clarify these issues.
In summary, our study shows a novel role of macrophage Hif-1α in mediating obesity-related adipose tissue dysfunction likely via an Irak-M dependent mechanism. Although the adipocyte is considered the key player in orchestrating local changes in the AT microenvironment during obesity, the current study adds to the emerging evidence on the important role for AT macrophages in such remodeling events.
GRANTS
JSP was supported by a scholarship from the Philippine Council for Health Research and Development, Department of Science and Technology, Republic of the Philippines, and a research grant from the Science, Technology, Research and Innovation for Development (STRIDE) program funded by the U.S. Agency for International Development (USAID). This work was supported by grants from the American Heart Association/SDG grant (MNB) and the American Thoracic Society Research Foundation (MNB).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.M.S.P., M.N.B., S.B., M.A., J.B.N.J., and U.J.M. conceived and designed research; J.M.S.P., S.B., and M.A. performed experiments; J.M.S.P., M.N.B., S.B., T.D.E., and U.J.M. analyzed data; J.M.S.P., M.N.B., T.D.E., J.W.C., and U.J.M. interpreted results of experiments; J.M.S.P. and S.B. prepared figures; J.M.S.P. and U.J.M. drafted manuscript; J.M.S.P., M.N.B., S.B., M.A., J.B.N.J., T.D.E., J.W.C., and U.J.M. approved final version of manuscript; M.N.B., S.B., M.A., J.B.N.J., T.D.E., J.W.C., and U.J.M. edited and revised manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. R. Flavell of Yale University for the original Irak-M−/− mice and Dr. G.-H. Fong of University of Connecticut for the original Phd2flox/flox mice.
REFERENCES
- 1.Arner E, Mejhert N, Kulyté A, Balwierz PJ, Pachkov M, Cormont M, Lorente-Cebrián S, Ehrlund A, Laurencikiene J, Hedén P, Dahlman-Wright K, Tanti JF, Hayashizaki Y, Rydén M, Dahlman I, van Nimwegen E, Daub CO, Arner P. Adipose tissue microRNAs as regulators of CCL2 production in human obesity. Diabetes 61: 1986–1993, 2012. doi: 10.2337/db11-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballinger MN, Newstead MW, Zeng X, Bhan U, Horowitz JC, Moore BB, Pinsky DJ, Flavell RA, Standiford TJ. TLR signaling prevents hyperoxia-induced lung injury by protecting the alveolar epithelium from oxidant-mediated death. J Immunol 189: 356–364, 2012. doi: 10.4049/jimmunol.1103124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballinger MN, Newstead MW, Zeng X, Bhan U, Mo XM, Kunkel SL, Moore BB, Flavell R, Christman JW, Standiford TJ. IRAK-M promotes alternative macrophage activation and fibroproliferation in bleomycin-induced lung injury. J Immunol 194: 1894–1904, 2015. doi: 10.4049/jimmunol.1402377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berra E, Benizri E, Ginouvès A, Volmat V, Roux D, Pouysségur J. HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1α in normoxia. EMBO J 22: 4082–4090, 2003. doi: 10.1093/emboj/cdg392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science 294: 1337–1340, 2001. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- 6.Chen W, Saxena A, Li N, Sun J, Gupta A, Lee DW, Tian Q, Dobaczewski M, Frangogiannis NG. Endogenous IRAK-M attenuates postinfarction remodeling through effects on macrophages and fibroblasts. Arterioscler Thromb Vasc Biol 32: 2598–2608, 2012. doi: 10.1161/ATVBAHA.112.300310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng JC, Cheng G, Newstead MW, Zeng X, Kobayashi K, Flavell RA, Standiford TJ. Sepsis-induced suppression of lung innate immunity is mediated by IRAK-M. J Clin Invest 116: 2532–2542, 2006. doi: 10.1172/JCI28054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eubank TD, Roberts RD, Khan M, Curry JM, Nuovo GJ, Kuppusamy P, Marsh CB. Granulocyte macrophage colony-stimulating factor inhibits breast cancer growth and metastasis by invoking an anti-angiogenic program in tumor-educated macrophages. Cancer Res 69: 2133–2140, 2009. doi: 10.1158/0008-5472.CAN-08-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan Q, Mao H, Xie L, Pi X. Prolyl hydroxylase domain-2 protein regulates lipopolysaccharide-induced vascular inflammation. Am J Pathol 189: 200–213, 2019. doi: 10.1016/j.ajpath.2018.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujisaka S, Usui I, Ikutani M, Aminuddin A, Takikawa A, Tsuneyama K, Mahmood A, Goda N, Nagai Y, Takatsu K, Tobe K. Adipose tissue hypoxia induces inflammatory M1 polarity of macrophages in an HIF-1α-dependent and HIF-1α-independent manner in obese mice. Diabetologia 56: 1403–1412, 2013. doi: 10.1007/s00125-013-2885-1. [DOI] [PubMed] [Google Scholar]
- 11.Global BMI Mortality Coalition; Di Angelantonio E, Bhupathiraju ShN, Wormser D, Gao P, Kaptoge S, Berrington de Gonzalez A, Cairns BJ, Huxley R, Jackson ChL, Joshy G, Lewington S, Manson JE, Murphy N, Patel AV, Samet JM, Woodward M, Zheng W, Zhou M, Bansal N, Barricarte A, Carter B, Cerhan JR, Smith GD, Fang X, Franco OH, Green J, Halsey J, Hildebrand JS, Jung KJ, Korda RJ, McLerran DF, Moore SC, O’Keeffe LM, Paige E, Ramond A, Reeves GK, Rolland B, Sacerdote C, Sattar N, Sofianopoulou E, Stevens J, Thun M, Ueshima H, Yang L, Yun YD, Willeit P, Banks E, Beral V, Chen Z, Gapstur SM, Gunter MJ, Hartge P, Jee SH, Lam TH, Peto R, Potter JD, Willett WC, Thompson SG, Danesh J, Hu FB. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 388: 776–786, 2016. doi: 10.1016/S0140-6736(16)30175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, Wang ZV, Landskroner-Eiger S, Dineen S, Magalang UJ, Brekken RA, Scherer PE. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol 29: 4467–4483, 2009. doi: 10.1128/MCB.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harada K, Isse K, Sato Y, Ozaki S, Nakanuma Y. Endotoxin tolerance in human intrahepatic biliary epithelial cells is induced by upregulation of IRAK-M. Liver Int 26: 935–942, 2006. doi: 10.1111/j.1478-3231.2006.01325.x. [DOI] [PubMed] [Google Scholar]
- 14.Hill AA, Reid Bolus W, Hasty AH. A decade of progress in adipose tissue macrophage biology. Immunol Rev 262: 134–152, 2014. doi: 10.1111/imr.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hulsmans M, Geeraert B, De Keyzer D, Mertens A, Lannoo M, Vanaudenaerde B, Hoylaerts M, Benhabilès N, Tsatsanis C, Mathieu C, Holvoet P. Interleukin-1 receptor-associated kinase-3 is a key inhibitor of inflammation in obesity and metabolic syndrome. PLoS One 7: e30414, 2012. doi: 10.1371/journal.pone.0030414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG Jr. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292: 464–468, 2001. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 17.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292: 468–472, 2001. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 18.Jiang C, Qu A, Matsubara T, Chanturiya T, Jou W, Gavrilova O, Shah YM, Gonzalez FJ. Disruption of hypoxia-inducible factor 1 in adipocytes improves insulin sensitivity and decreases adiposity in high-fat diet-fed mice. Diabetes 60: 2484–2495, 2011. doi: 10.2337/db11-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Magalang UJ, Cruff JP, Rajappan R, Hunter MG, Patel T, Marsh CB, Raman SV, Parinandi NL. Intermittent hypoxia suppresses adiponectin secretion by adipocytes. Exp Clin Endocrinol Diabetes 117: 129–134, 2009. doi: 10.1055/s-2008-1078738. [DOI] [PubMed] [Google Scholar]
- 21.Marxsen JH, Stengel P, Doege K, Heikkinen P, Jokilehto T, Wagner T, Jelkmann W, Jaakkola P, Metzen E. Hypoxia-inducible factor-1 (HIF-1) promotes its degradation by induction of HIF-alpha-prolyl-4-hydroxylases. Biochem J 381: 761–767, 2004. doi: 10.1042/BJ20040620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazzone M, Dettori D, de Oliveira RL, Loges S, Schmidt T, Jonckx B, Tian YM, Lanahan AA, Pollard P, de Almodovar CR, De Smet F, Vinckier S, Aragonés J, Debackere K, Luttun A, Wyns S, Jordan B, Pisacane A, Gallez B, Lampugnani MG, Dejana E, Simons M, Ratcliffe P, Maxwell P, Carmeliet P. Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell 136: 839–851, 2009. doi: 10.1016/j.cell.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramkhelawon B, Hennessy EJ, Ménager M, Ray TD, Sheedy FJ, Hutchison S, Wanschel A, Oldebeken S, Geoffrion M, Spiro W, Miller G, McPherson R, Rayner KJ, Moore KJ. Netrin-1 promotes adipose tissue macrophage retention and insulin resistance in obesity. Nat Med 20: 377–384, 2014. doi: 10.1038/nm.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rio DC, Ares M Jr., Hannon GJ, Nilsen TW. Purification of RNA using TRIzol (TRI reagent). Cold Spring Harb Protoc 2010: pdb prot5439, 2010. 10.1101/pdb.prot5439. [DOI] [PubMed] [Google Scholar]
- 25.Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell 156: 20–44, 2014. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shalova IN, Lim JY, Chittezhath M, Zinkernagel AS, Beasley F, Hernández-Jiménez E, Toledano V, Cubillos-Zapata C, Rapisarda A, Chen J, Duan K, Yang H, Poidinger M, Melillo G, Nizet V, Arnalich F, López-Collazo E, Biswas SK. Human monocytes undergo functional re-programming during sepsis mediated by hypoxia-inducible factor-1α. Immunity 42: 484–498, 2015. doi: 10.1016/j.immuni.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Sun K, Halberg N, Khan M, Magalang UJ, Scherer PE. Selective inhibition of hypoxia-inducible factor 1α ameliorates adipose tissue dysfunction. Mol Cell Biol 33: 904–917, 2013. doi: 10.1128/MCB.00951-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest 121: 2094–2101, 2011. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun K, Tordjman J, Clément K, Scherer PE. Fibrosis and adipose tissue dysfunction. Cell Metab 18: 470–477, 2013. doi: 10.1016/j.cmet.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun K, Wernstedt Asterholm I, Kusminski CM, Bueno AC, Wang ZV, Pollard JW, Brekken RA, Scherer PE. Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proc Natl Acad Sci USA 109: 5874–5879, 2012. doi: 10.1073/pnas.1200447109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi N, Honda T, Domon H, Nakajima T, Tabeta K, Yamazaki K. Interleukin-1 receptor-associated kinase-M in gingival epithelial cells attenuates the inflammatory response elicited by Porphyromonas gingivalis. J Periodontal Res 45: 512–519, 2010. doi: 10.1111/j.1600-0765.2009.01266.x. [DOI] [PubMed] [Google Scholar]
- 32.Takeda K, Ho VC, Takeda H, Duan LJ, Nagy A, Fong GH. Placental but not heart defects are associated with elevated hypoxia-inducible factor alpha levels in mice lacking prolyl hydroxylase domain protein 2. Mol Cell Biol 26: 8336–8346, 2006. doi: 10.1128/MCB.00425-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takikawa A, Mahmood A, Nawaz A, Kado T, Okabe K, Yamamoto S, Aminuddin A, Senda S, Tsuneyama K, Ikutani M, Watanabe Y, Igarashi Y, Nagai Y, Takatsu K, Koizumi K, Imura J, Goda N, Sasahara M, Matsumoto M, Saeki K, Nakagawa T, Fujisaka S, Usui I, Tobe K. HIF-1α in myeloid cells promotes adipose tissue remodeling toward insulin resistance. Diabetes 65: 3649–3659, 2016. doi: 10.2337/db16-0012. [DOI] [PubMed] [Google Scholar]
- 34.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112: 1796–1808, 2003. doi: 10.1172/JCI200319246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang P, Cao L, Zhou R, Yang X, Wu M. The lncRNA Neat1 promotes activation of inflammasomes in macrophages. Nat Commun 10: 1495, 2019. doi: 10.1038/s41467-019-09482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages. Curr Protoc Immunol 83: 14.1.1–14.1.14, 2008. doi: 10.1002/0471142735.im1401s83. [DOI] [PMC free article] [PubMed] [Google Scholar]