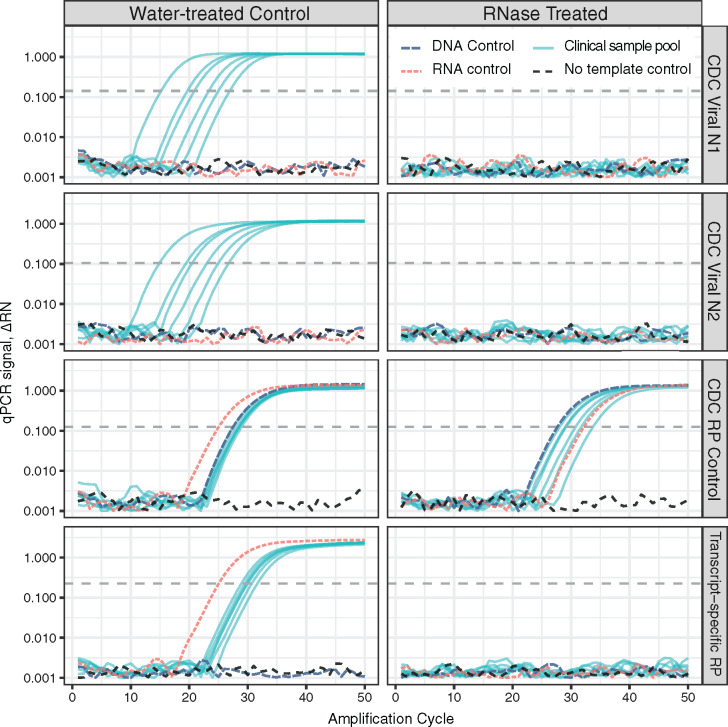
Fig. 1.
The de facto standard COVID-19 RT-qPCR test design includes a specimen and extraction control that is DNA cross-reactive and generates analytical false-negative results following loss of RNA integrity. Purified nucleic acid samples from COVID-19 positive cases were pooled, aliquoted, and treated with either nuclease-free water or ribonuclease A (RNase). Commercial human RNA, DNA, and no-template controls were treated in parallel. Samples were analyzed by RT-qPCR with Viral One-step master mix using the CDC-specified Viral N1, Viral N2, and human specimen and extraction control (RP) assays, plus a transcript-specific control targeting spliced exons 1–3 of RPP30 (Hs.PT.58.2854947, idtdna.com). Strong viral and specimen control signals are generated from each infected-patient pool after mock treatment (water, left). Purified DNA is sufficient template for the CDC RP control. Loss of RNA integrity precludes detection of viral genomes, while the cross-reactive specimen control continues to generate strong signal from copurified DNA (RNase, right). A transcript-specific RP control properly reflects the loss of sample integrity and returns no signal from samples containing degraded RNA.