Sir,
Healthcare workers (HCWs) are a high-risk population for SARS-CoV-2 infection and account for at least 11% of reported cases.1,2
We performed an observational cross-sectional case-control study to evaluate the efficacy of hydroxychloroquine pre-exposure prophylaxis (PrEP) among hospital HCWs.
All HCWs (of all categories) who worked in the COVID-19 frontline wards of University Hospital Germans Trias (Badalona, Spain) were invited to participate. There was no protocol in the institution recommending hydroxychloroquine PrEP. The dose used by HCWs was 400 mg twice daily on the first day and 200 mg twice daily for an additional 4 days, with a maintenance dosing of 200 mg weekly thereafter.
HCWs were classified as having high-risk occupational exposure if they worked in hospital-based COVID-19 wards, moderate-risk occupational exposure if they had direct contact with admitted patients, but not in COVID-19 wards, and low-risk occupational exposure if they had occasional contact with hospitalized patients.
Reverse real-time PCR was performed for nasopharyngeal swabs of all HCWs with symptoms or suspicion of COVID-19. A screening of SARS-CoV-2 serology of all hospital HCWs was performed when the epidemic reached its end (late May 2020).
The study was approved by the Institutional Review Board (PI-20–171) and participants provided written informed consent.
All analyses were adjusted for age, sex, occupational exposure risk and type of HCW. We performed a propensity-score matching with the algorithm of nearest neighbour matching using the R Optmatch package. All analyses were repeated using SARS-CoV-2 antibodies as a diagnostic tool.
We identified 69 HCWs receiving hydroxychloroquine PrEP and compared them with 418 HCWs who did not, working in the same hospital and period (Table S1, available as Supplementary data at JAC Online).
Overall, 81 (16.63%) were diagnosed with COVID-19 by nasopharyngeal reverse real-time PCR and 79/464 (17.03%) after the epidemic for anti-SARS-CoV-2-IgG antibodies. No subject received antiviral or immunomodulatory treatment.
The crude rates of SARS-CoV-2 infection with (versus without) hydroxychloroquine PrEP were, respectively, 23.19% (16/69) versus 15.55% (65/418) by reverse real-time PCR and 28.33% (17/60) versus 15.35% (62/404) by serology.
The median (IQR) time from hydroxychloroquine PrEP initiation to COVID-19 diagnosis by reverse real-time PCR was 14 (7–23) days.
The rates of SARS-CoV-2 infection assessed by reverse real-time PCR among those on hydroxychloroquine PrEP stratified by their risk (high, moderate and low) of exposure to patients admitted with COVID-19 were 22.92%, 22.50% and 15.33%, respectively. The corresponding rates were 23.81%, 15.79% and 16.41%, respectively, by serology.
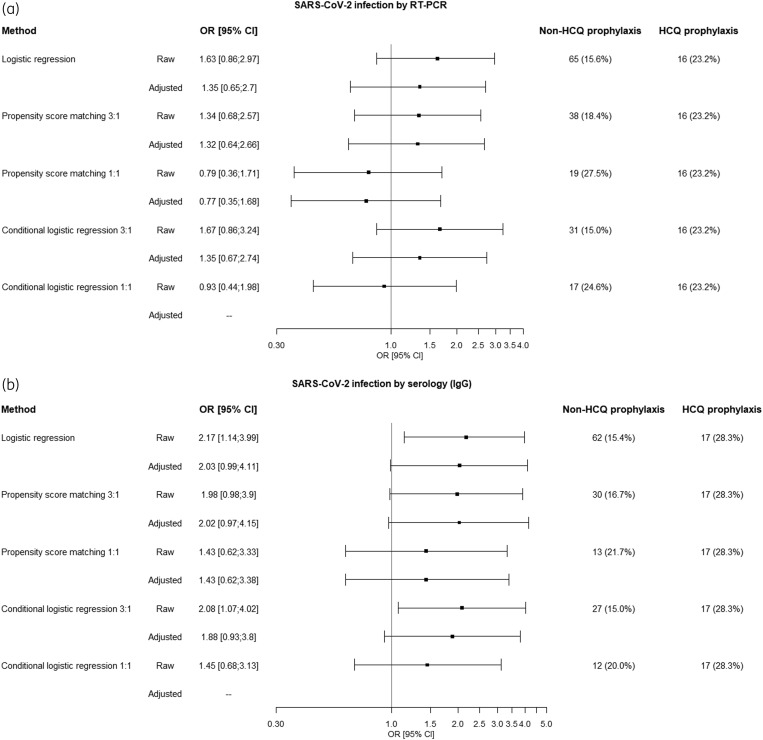
A propensity-score matching with a ratio of experimental-to-control subjects of 1:3 did not culminate in optimal balancing. A propensity-score analysis with 1:1 matching led to a complete adjustment. The corresponding OR (PrEP versus non-PrEP) was 0.77 (95% CI = 0.35–1.68) by reverse real-time PCR and 1.43 (95% CI = 0.62–3.38) by serology (Figure 1).
Figure 1.
Compilation of ORs for COVID-19 infection in HCWs receiving hydroxychloroquine PrEP for COVID-19, assessed by either reverse real-time PCR (a) or by SARS-CoV-2 antibodies (b).
In hospital HCWs, hydroxychloroquine PrEP did not prevent confirmed COVID-19, diagnosed by either reverse real-time PCR or by serology afterwards. Results were concordant using an adjusted logistic regression, a propensity-score matched case-control analysis or a full matching algorithm nested in a cohort of HCWs from the same institution and period. The model showed a complete adjustment with absolute standardized differences below 10%, demonstrating that covariate imbalance did not remain after matching.3
The OR values in our study were consistently >1 for COVID-19 infection among those receiving hydroxychloroquine PrEP and, in some analyses, we even found an increased risk of infection, particularly when assessed by SARS-CoV-2 antibodies (Figure 1).
Previous reports have identified that hydroxychloroquine inhibits trained immunity and reduces expression of IFN-stimulated genes,4 suggesting that the drug might not have a beneficial effect on the antiviral immune response to SARS-CoV-2. Moreover, research in Chikungunya infection has found enhanced infection following chloroquine treatment in non-human primates and in a human cohort.5 Though our results are very robust in the identification of an absence of PrEP efficacy of hydroxychloroquine, the possibility of increasing the risk of infection is not concordant and the interpretation must be very cautious.
The Recovery trial has reported no benefit in hospitalized patients with COVID-19.6 Another randomized study found no benefit in early treatment.7 On 15 July 2020, the FDA revoked the hydroxychloroquine Emergency Use Authorization. Lastly, a randomized clinical trial has recently shown no hydroxychloroquine efficacy in post-exposure prophylaxis.8
However, there are still many ongoing studies assessing the efficacy of hydroxychloroquine PrEP against COVID-19 in ClinicalTrials.gov, some of them that are going to expose a high number of HCWs (HERO-HCQ and COPCOV COVID-19 trials among them) to this strategy.
The rate of SARS-CoV-2 antibodies in the predefined high-risk hospital departments, 17.03%, is significantly higher than the 9.41% found in the whole hospital staff of the same centre and time period (adjusted difference = +7.62, 95% CI = 4.13–11.09, P < 0.0001).9 The corresponding rate in the general population in the same province and period was 7.0% and among HCWs in another hospital in the same metropolitan area the rate was 11.2%.2,10 Therefore, there is an urgent need to discover effective PrEP strategies for high-risk HCWs.
Our study has limitations, including its retrospective design. We performed, however, a propensity-score adjustment for a number of COVID-19-relevant confounders that achieved a complete adjustment. Despite this, we cannot rule out residual confounding. We screened all HCWs by serology, allowing us a more accurate estimate of COVID-19 infection among HCWs, including those who remained undiagnosed during the epidemic.
In conclusion, this study did not demonstrate a significant benefit of hydroxychloroquine PrEP for COVID-19 prevention in hospital HCWs. These results support the implementation of early safety and efficacy controls in ongoing trials assessing hydroxychloroquine PrEP by Data and Safety Monitoring Boards, considering that the drug has failed in COVID-19 treatment overall, in early treatment, in post-exposure prophylaxis and this analysis suggests the same could happen in PrEP as well.
Supplementary Material
Acknowledgements
We are grateful to Cristina Rodriguez and Antoni Jou for their tireless work in data managing. We acknowledge all healthcare workers involved in this pandemic in the frontline at the University Hospital Germans Trias in Badalona, Spain.
Funding
This research was partly funded via the crowdfunding campaign #Yomecorono (https://www.yomecorono.com/), Barcelona, Spain, with the contribution of over 72 000 citizens and corporations. This research was also partially supported by Agencia Estatal de Investigación-Ministerio de Ciencia e Innovación (grants PID2019-104830RB-I00 and MTM2015‐64465‐C2‐1‐R, MINECO/FEDER) and Generalitat de Catalunya (2017 SGR 622).
Transparency declarations
None to declare.
Author contributions
B.R., B.C. and J.M.L. conceived the project. C.T., J.P. and N.P.-A. performed statistical analyses. J.M.L. wrote the paper. All authors had the opportunity to discuss the results and comment on the manuscript. C.T. and J.P. produced Figure 1. Critical revision of the manuscript for important intellectual content was performed by B.R., B.C., S.V. and C.T.
Supplementary data
Table S1 is available as Supplementary data at JAC Online.
References
- 1. CDC COVID-19 Response Team. Characteristics of health care personnel with COVID-19—United States, February 12–April 9, 2020. MMWR Morb Mortal Wkly Rep 2020; 69: 477–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Garcia-Basteiro AL, Moncunill G, Tortajada M. et al. Seroprevalence of antibodies against SARS-CoV-2 among health care workers in a large Spanish reference hospital. Nat Commun 2020; 11: 3500.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morgan CJ. Reducing bias using propensity score matching. J Nucl Cardiol 2018; 25: 404–6. [DOI] [PubMed] [Google Scholar]
- 4. Rother N, Yanginlar C, Lindeboom RGH. et al. Hydroxychloroquine inhibits trained immunity - implications for COVID-19. medRxiv 2020; doi:10.1101/2020.06.08.20122143. [Google Scholar]
- 5. Guastalegname M, Vallone A. Could chloroquine/hydroxychloroquine be harmful in coronavirus disease 2019 (COVID-19) treatment? Clin Infect Dis 2020; 71: 888–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. RECOVERY Collaborative Group. Effect of hydroxychloroquine in hospitalized patients with COVID-19: preliminary results from a multi-centre, randomized, controlled trial. MedRxiv 2020; doi:10.1101/2020.07.15.20151852. [Google Scholar]
- 7. Mitjà O, Corbacho-Monné M, Ubals M. et al. Hydroxychloroquine for early treatment of adults with mild Covid-19: a randomized-controlled trial. Clin Infect Dis 2020; doi:10.1093/cid/ciaa1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boulware DR, Pullen MF, Bangdiwala AS. et al. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N Engl J Med 2020; 383: 517–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barallat J, Fernández-Rivas G, Quirant-Sánchez B. et al. Seroprevalence of SARS-CoV-2 IgG specific antibodies among healthcare workers in the Northern Metropolitan Area of Barcelona, Spain, after the first pandemic wave. medRxiv 2020; doi:10.1101/2020.06.24.20135673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pollán M, Pérez-Gómez B, Pastor-Barriuso R. et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet 2020; 396: 535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.