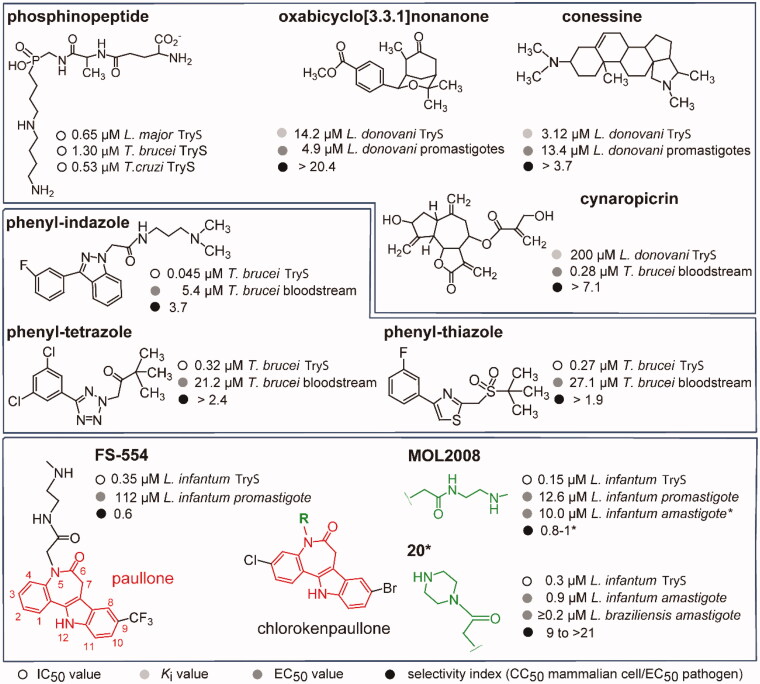
Figure 1.
Trypanothione synthetase inhibitors. The structure and biological activities (IC50 against TryS or Ki, EC50 against trypanosomatids and selectivity index) are shown for representatives singletons or scaffolds reported to inhibit TryS. The data shown in the upper box were reported in14 for phosphinopeptides, in19 for oxabicyclo nonanone, in15 for conessine, and in16 for cynaropicrin. Data shown in the middle box was reported in17 for the phenyl-indazole derivative and in18 for the phenyl-tetrazole and -thiazole derivatives. Lower panel, the 7,12-dihydroindolo[3,2-d][1]benzazepin-6(5H)-ones core scaffold, referred as paullone, is shown in red. Incorporation of a Cl and Br atom at position 3 and 9, respectively, give rise to chlorokenpaullone. The asterisk denotes data reported in this work whereas information for FS-554 {9-trifluoromethylpaullone with N-[2-(methylamino)ethyl]acetamide side chain} and MOL2008 {3-chlorokenpaullone with N-[2-(methylamino)ethyl]acetamide side chain} was reported elsewhere10,20. Compound 20 is a 3-chlorokenpaullone with 2-oxo-2-piperazinoethyl side chain as hydrochloride.