Figure 1. Recruitment and dynamics of vascular and perivascular monocyte/macrophages.
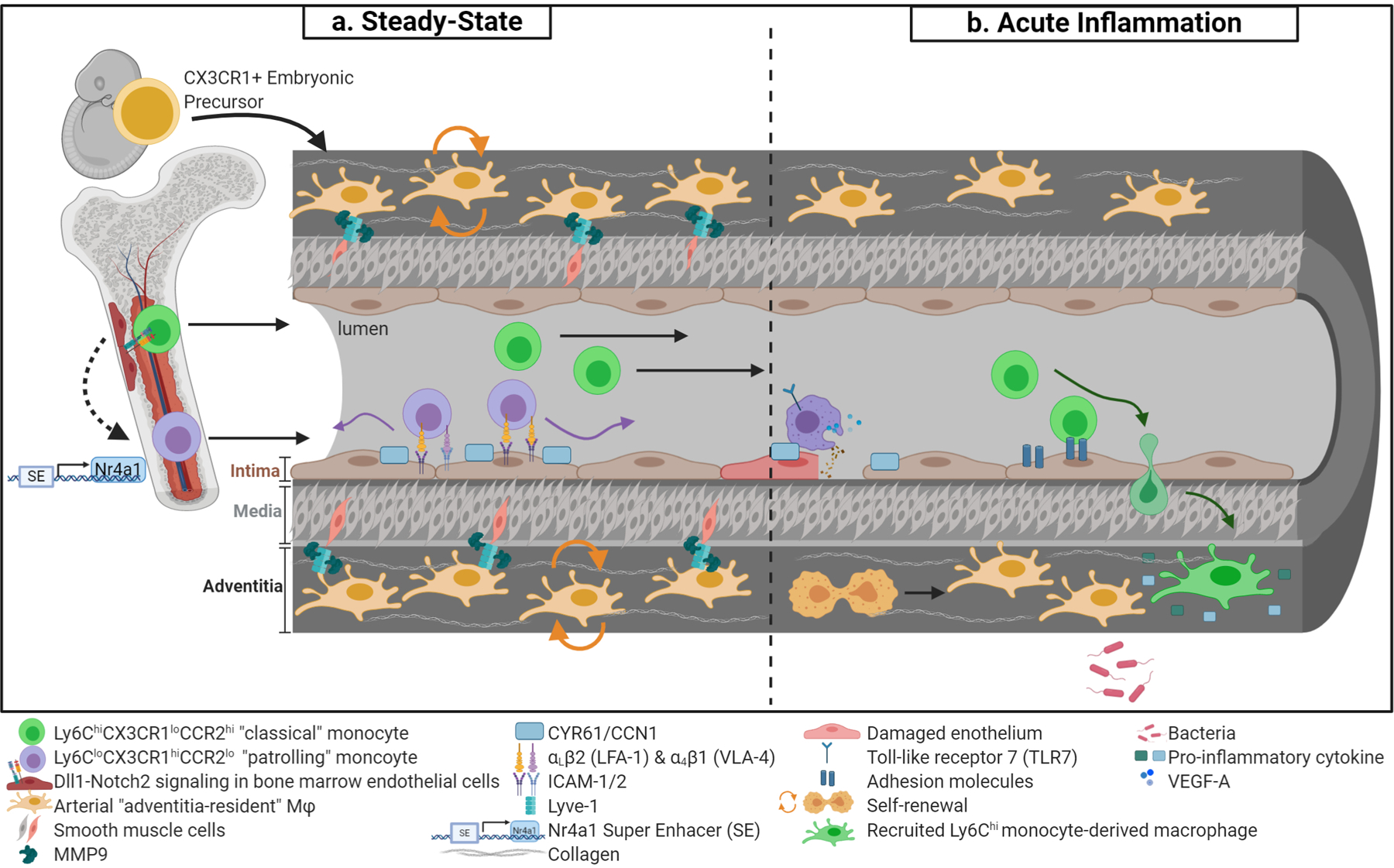
(a) Arterial “adventitia-resident” macrophages are derived from CX3CR1+ embryonic precursors and seed the perivasculature prior to birth (orange cells). Throughout adulthood, adventitia-resident macrophages are maintained by self-renewal. Adventitia-resident macrophages regulate collagen content via matrix metalloproteinase-p (Mmp9) proteolysis, which is dependent on Lyve-1 and smooth muscle cell binding. At stead-state, Ly6Chi classical monocytes (in green) are the precursors to Ly6Clo patrolling monocytes (in purple). Monocyte conversion is dependent on Dll1/Notch2 signaling in distinct vascular beds. Additionally, Nr4a1 super enhancer regulates patrolling monocyte development and is essential for monocyte survival. In the circulation, patrolling monocytes crawling requires αLβ2 integrin (or LFA-1) and other adhesion molecules highlighted. Furthermore, endothelial cystein-rich angiogenic inducer 61 (Cyr61 or Ccn1) promotes efficient surveillance. (b) Patrolling monocytes remove cellular debris from the endothelial surface at sites of necrosis in a TLR-7-dependent manner. At sites of injured endothelium, patrolling monocytes may facilitate endothelial proliferation through secretion of Vegfa. Moreover, activated endothelium upregulates adhesion molecules that enable monocyte capture, rolling, and extravasation into tissue and migrate to inflammatory stimuli. Recruited monocytes differentiate into macrophages as part of a pro-inflammatory response. Adventitia-resident macrophages are severely depleted during bacterial infection but are able to regain steady-state levels through self-renewal.
