Supplemental Digital Content is available in the text
Keywords: HIV-1, Immune reconstitution, Immunological non-responders, Immunotherapy, Natural killer cell, NK cell
Abstract
Background:
Allogeneic natural killer (NK) cell immunotherapy is recognized as a promising anti-tumor strategy, but whether it plays a role in poor CD4 recovery among human immunodeficiency virus type 1 (HIV-1) infected patients is unknown. This study aimed to investigate the safety and effectiveness of allogeneic NK cells immunotherapy on HIV-1 immunological non-responders (INRs) receiving antiretroviral therapy (ART).
Methods:
From February to April 2018, a prospective, randomized, controlled, open-label clinical trial, which enrolled 20 HIV-1 INRs following specific inclusion criteria, was conducted at Nankai University Second People's Hospital. Participants were randomly allocated (simple randomization 1:1) to either the combined treatment (NK + ART) group (n = 10) or the control (ART) group (n = 10). The allogenic highly activated NK cells from killer cell immunoglobulin-like receptor (KIR)/human leukocyte antigen (HLA)-Cw mismatched healthy donor were prepared (108 cells in each injection) and intravenously infused to each recruited patient of NK+ART group in three courses. Key immune parameters (CD4 count, CD8 count, CD4/CD8 ratio), laboratory tests (count of blood cells, biochemistry panel) and symptoms at baseline and at month 1, 3, 6, 9, 12, and 24 were measured/collected to analyze the safety and efficacy of the therapy. Comparisons were between the seven time-points of both groups using repeated measurement analysis of variance (ANOVA) test. Generalized estimating equations (GEE) model was performed to evaluate the overall effect of the NK+ART group vs. the ART group.
Results:
From baseline to 24 months, we noted a mean CD4 count augmentation (139 to 243 cells/μL) in the NK + ART group and (144 to 176 cells/μL) in the ART group (difference, 67; 95% CI, 10 to 124; P = 0.024). Our estimations revealed that NK+ART group could improve CD4 level (β = 54.59, P = 0.006) and CD8 level (β = 322.47, P = 0.010) on average among the six measurements compared with the ART group. Only two (2/10, 20%) participants in the NK+ART group developed a transient mild fever after the first course.
Conclusions:
This preliminary study informs that HIV-1 INRs, allogenic NK cells immunotherapy is safe and could significantly improve CD4 recovery but not CD4/CD8 ratio. The practical effects, however, need long-term follow-up observations. Further study on the potential underlying mechanism is warranted.
Registration info:
www.chictr.org.cn/showproj.aspx?proj=34912 (No. ChiCTR1900020634).
Introduction
Despite successful viral suppression under antiretroviral therapy (ART), approximately 10% to 40% of human immunodeficiency virus type 1 (HIV-1) patients fail to reconstitute their CD4+ T-cell counts to similar levels of healthy controls.[1] Immunological non-responders (INRs) are usually defined as patients with peripheral CD4 counts < 200 cells/μL in 2 years after ART initiation, with an undetectable plasma viral load.[2,3] The potential mechanisms of inadequate immune reconstitution are unclear; the main factors involved are low nadir CD4 count, older age, reduced thymic output, aberrant immune activation, residual virus replication, and so on.[1] Immunological recovery failure associates with increased morbidity and mortality in HIV-1 positive patients and currently lacks effective treatment.[1] Allogeneic-activated natural killer (NK) cells immunotherapy has presently reported as being a promising anti-tumor strategy, ranging from hematological malignancies to various types of solid tumors.[4,5] Most of the NK cells exhibit efficient anti-tumor cytolytic activity by recognizing non-self without prior activation or immunization.[5] Uniformly, NK cells represent critical innate immune cells that can rapidly recognize and kill virally infected cells. However, whether allogeneic NK cells immunotherapy plays a role in poor CD4 recovery among RNA suppressed ART-treated HIV-1 infected patients is unknown. Then we conducted this cellular trial using allogeneic NK cells to investigate their safety and clinical efficacy in the treatment of HIV-1 INRs.
Methods
Ethical approval
The Human Medical Ethics Committee of Tianjin Second People's Hospital approved the study (No. 2018-27), which was registered at the Chinese Clinical Trial Registry (No. ChiCTR1900020634). Written informed consent was provided by each participant.
Study participants
Between February 2018 and April 2018, 20 HIV-1 patients were enrolled and randomly assigned (simple randomization 1:1) either in the allogeneic NK cells immunotherapy combined with ART group (NK + ART) (n = 10) or the control (ART) group (n = 10). The inclusion criteria were as follows: (1) 18 to 65 years old; (2) stable ART for at least 2 years; (3) CD4 count < 200 cells/μL in 2 years after ART initiation; (3) plasma HIV RNA < 20 copies/mL. Exclusion criteria were as listed: (1) history of prior malignancies; (2) active opportunistic infections within 4 months prior to recruitment; (3) Active substance abuse; (4) pregnant or breastfeeding women; (5) allergic constitution; (6) critically illness; (7) any psychiatric or medical condition that might influence the study according to the investigator.
Cell products preparation
Allogenic NK cells were prepared under good manufacturing practice (GMP) conditions in a certified laboratory.[6] The donors were selected based on DNA genotyping of their human leukocyte antigen (HLA)-Cw mismatch with the killer cell immunoglobulin-like receptor (KIR) of HIV-1 patients, and their peripheral blood mononuclear cells (PBMC) were collected. KIR/HLA-Cw mismatch was defined as the absence of one or more HLA alleles known to be ligands for the inhibitory KIR typing, based on previously published criteria.[7] The human NK cells in vitro culture synergistic kit (HAHK Bioengineering Co. Ltd., Shenzhen, China) was used according to the manufacturer's instructions, with X-VIVO 15 serum-free medium (Lonza, Walkersville, MD, USA), interleukin (IL)-2, and membrane chimeric cellular factors including IL-21 which co-stimulate expansion and activation of NK cells in the PBMC for about 14 days. The NK cells were then purified by a magnetic cell separator (Miltenyi Biotec, Germany). The final products were used for the infusion. The details of the method and cell product profile have already been published.[6]
NK therapy procedure
The patients in the NK + ART group received continuous treatment of three courses of NK cells immunotherapy, three consecutive days for each course, and the interval of two courses was 1 month. After each infusion of 108 cells, the participants were observed for 3 h to monitor their vital signs (blood pressure, heart rates) and the onset of eventual symptom was reported. Then, the participants were followed-up with successive visits at 1, 3, 6, 9, 12, and 24 months after the last injection of NK cells. On each subsequent visit, peripheral blood was sampled to detect HIV viral load, determine CD4 count, establish a complete blood count, and evaluate the biochemical blood index. The ART group did not receive NK cell products as it serves as a control to evaluate the effects of the intervention.
Safety and efficacy evaluation
The primary outcome was CD4+ T-cell count at 24 months after NK cells treatment. The secondary outcomes were represented by two other parameters recorded at 24 months after NK cells infusion; they were: CD8+ T-cell count and CD4/CD8 ratio.
Adverse events (AEs) during the trial and post-treatment were recorded. All AEs were evaluated in accordance with the Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0.[8]
Statistical analysis
Categorical variables were reported as frequencies (percentages); continuous variables were presented as median (interquartile range) or mean ± standard deviation as appropriate. Differences in categorical variables were assessed using the chi-square test or Fisher exact test. Continuous variables were compared using t-tests or Mann-Whitney U test. Comparisons were between the seven time-points of both groups using repeated measurement analysis of variance (ANOVA) test. Generalized estimating equations (GEE) model was performed to evaluate the overall effect of the NK+ART group vs. the ART group, we hypothesized that correlation depends on interval of time between responses, so autoregressive (AR) (1) correlation matrix was used. A two-tailed P value < 0.05 was considered statistically significant. All analyses were performed using SAS version 9.4 software (SAS Institute, Cary, NC, USA).
Results
Profile of the participants
There were 20 patients meeting the inclusion criteria that were willing to participate in the trial. Ten were allocated to NK + ART group and 10 to the control group. As shown in Table 1, the two groups were comparable in terms of sex, age, CD4+ T-cell count, nadir CD4, CD8+ T-cell count, CD4/CD8 ratio, plasma HIV RNA, and ART duration.
Table 1.
Clinical characteristics of all participants with human immunodeficiency virus type 1.
| NK + ART | ART | |||
| Variable | (n = 10) | (n = 10) | Z | P |
| Female/male, n | 0/10 | 0/10 | – | – |
| Age (years) | 56 (53–58) | 55 (47–62) | <0.001 | >0.999 |
| CD4+ T-cell count (cells/μL) | 151 (110–178) | 124 (118–192) | 0.340 | 0.733 |
| Nadir CD4 count (cells/μL) | 18 (7–69) | 27 (17–37) | 0.340 | 0.734 |
| CD8+ T-cell count (cells/μL) | 586 (270–965) | 642 (441–787) | 0.794 | 0.427 |
| CD4/CD8 ratio | 0.24 (0.16–0.41) | 0.24 (0.17–0.32) | 0.643 | 0.521 |
| Plasma HIV RNA (copies/mL) | Undetectable | Undetectable | – | – |
| Years of ART | 3.58 (2.51–5.69) | 2.47 (2.02–3.81) | –1.323 | 0.186 |
Data expressed as median (interquartile ranges) or n. –: Not applicable. ART: Antiretroviral therapy; NK: Natural killer cell therapy; HIV: human immunodeficiency virus.
Efficacy
The mean CD4 count from baseline to 24 months increased from 139 to 243 cells/μL in the NK + ART group and from 144 to 176 cells/μL in the ART group (difference, 67; 95% CI, 10 to 124; P = 0.024). The mean CD8 count from baseline to 24 months increased from 637 to 839 in the NK + ART group but decreased from 771 to 541 in the ART group (difference, 298; 95% CI, −61 to 657; P = 0.098). There were no significant differences between the NK + ART group and the ART group in the CD4/CD8 ratio level (0.34 vs. 0.38; difference, –0.04; 95% CI, –0.19 to 0.11; P = 0.569) assessed at 24 months [Figure 1A–C].
Figure 1.
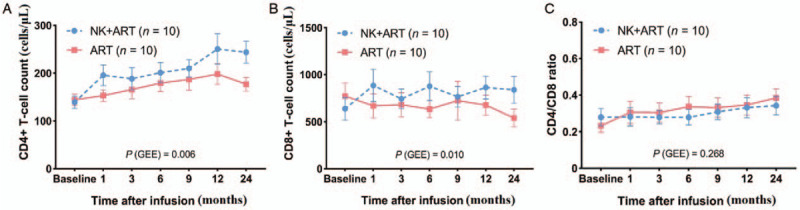
Evolution of the considered markers throughout the follow-up. Mean CD4+ T-cell count (A), CD8+ T-cell count (B), and CD4/CD8 ratio (C) progressions in the NK + ART group vs. ART group. ART: Antiretroviral therapy; GEE: Generalized estimating equations; NK: Natural killer cell therapy.
Considering the correlated nature of the repeated measurement responses, GEE and AR correlation matrix were used. After adjusting for baseline CD4+ T-cell count, baseline CD8+ T-cell count, and age; we estimated that the NK+ART group could improve CD4 level (β = 54.59, P = 0.006) and CD8 level (β = 322.47, P = 0.010) on average among the six measurements compared with the ART group [Table 2].
Table 2.
Evaluation of the effect of NK infusions on CD4 count, CD8 count, and CD4/CD8 ratio using GEE model.
| CD4+ T-cell count | CD8+ T-cell count | CD4/CD8 ratio | ||||
| Variable | β (95% CI) | P | β (95% CI) | P | β (95%CI) | P |
| NK+ART vs. ART | 54.59 (15.98, 93.21) | 0.006 | 322.47 (76.15, 568.7) | 0.010 | –0.06 (–0.16, 0.04) | 0.268 |
| baseline CD4 count | 0.54 (0.03, 1.06) | 0.038 | 2.66 (–0.30, 5.62) | 0.079 | 0.0004 (–0.0012, 0.0019) | 0.647 |
| baseline CD8 count | 0.02 (–0.04, 0.07) | 0.518 | 0.54 (0.16, 0.92) | 0.006 | –0.0002 (–0.0003, –0.0000) | 0.016 |
| Age | –1.62 (–3.40, 0.16) | 0.075 | –9.24 (–21.99, 3.51) | 0.155 | 0.0011 (–0.0036, 0.0059) | 0.645 |
Adjusted for baseline CD4+ T-cell count, baseline CD8+ T-cell count and Age. ART: Antiretroviral therapy; CI: Confidence interval; GEE: Generalized estimating equations; NK: Natural killer.
Safety and adverse events
Quantitative value of blood cells including white cell count, lymphocyte, red cell count, hemoglobin, and platelet were compared between the two groups. The result revealed no significant difference between the two groups at any of the seven time-points considered. Further, biological constants related to liver function (measured as alanine aminotransferase, aspartate aminotransferase, and total bilirubin) and renal function (measured as creatine, estimated glomerular filtration rate) were not statistically different either [Supplementary Table 1, http://links.lww.com/CM9/A374].
All participants were followed up for AEs. Two (2/10, 20%) participants in the NK+ART group developed a transient mild fever after the 1st course of the trial, which was thought to be unexpected study-related adverse events. None of the participants developed severe AEs (leading to discontinuation of the trial or hospitalization) or died during the treatment and post-treatment. No relapse of HIV-1 viremia observed during the observation period.
Discussion
Poor CD4+ T cell recovery on suppressive antiretroviral therapy represents a major clinical issue. Thus far, many intervention measures have been used to improve the level of immune reconstitution, such as Vit D supplementation,[9] intensification with raltegravir or maraviroc,[1,10] and utilization of some immunomodulatory agents to limit immune activation.[11] However, these measures have not yield convincing results. To our knowledge, the impact of allogeneic NK cell immunotherapy on the restoration of CD4 count of HIV-1 immunologic non-responders has seldom been reported. In this preliminary study the patients receiving allogeneic NK cell immunotherapy, compared to the control group, did significantly improve immune reconstitution (as measured by the CD4 count at 24 months after NK cells infusion).
It is generally accepted that KIR/HLA-incompatible NK cells are more efficient in treating different cancer settings and appear not to provoke graft-versus-host response.[5,12] In our study, two participants got a fever after the infusion of NK cells, which was viewed as mild adverse events. These preliminary observations indicate that allogeneic NK cell immunotherapy in HIV-1 individuals is safe and well-tolerated.
The absolute CD4 and CD8 counts increased from baseline to 24 months after NK cells infusion, but the NK cell therapy did not show a beneficial effect on CD4/CD8 ratio gain. Despite adequate viral suppression and improvement of CD4 levels, the high levels of CD8+ T cells are maintained, and the CD4/CD8 ratio fails to restore.[13,14] A previous study showed that if ART initiated during the chronic HIV-1 infection stage, most of the patients would fail to normalize CD4/CD8 ratio after more than 10 years of viral suppression and recovery of CD4 levels to >500.[15] Then, viral suppression and CD4+ T-cell response under ART therapy will consistently remain key therapeutic goals in HIV-1 management. Failure to improve the CD4/CD8 ratio in our study may be due to the initiation of ART during chronic HIV.
This study has several limitations. First, by including a limited number of patients we could not adequately draw exact conclusions on the effect of allogeneic NK cells on patient outcome. Second, other immunological (eg, immune activation, immune exhaustion) markers were not analyzed; having them tested may provide evidence to support our conclusion. Also, the time of HIV-1 infection which was not considered during the participants’ selection and distribution into different groups could be a factor limiting the interpretation of our results. Blood samples were collected and cryopreserved from all the participants. Further immunologic research should be conducted in order to explain the possible mechanisms hidden behind the role of allogeneic NK cells in CD4 recovery in HIV-1 patients.
In summary, our study demonstrates the use of allogeneic NK cells immunotherapy strategy as a possible treatment approach for HIV-1 immunological non-responders. Future validation studies with a larger number of participants are required. Meanwhile, the current study could represent important evidence leading to the improvement of future strategies regarding the management of HIV-1 immunological non-responders.
Funding
This study was funded by the Key Project of Tianjin Second People's Hospital (No. YS0001), the National Natural Science Foundation of China (No. 82002136), and the 13th Five-year National Major Project for HIV/AIDS and Hepatitis B Control and Prevention, and the Chinese Ministry of Science and Technology (Nos. 2017ZX10202102005004, 2018ZX10302104-002).
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Xia H, Wang Y, Sun HL, Gao LY, Cao Y, Zaongo SD, Zeng RN, Wu H, Zhang MJ, Ma P. Safety and efficacy of allogeneic natural killer cell immunotherapy on human immunodeficiency virus type 1 immunological non-responders: a brief report. Chin Med J 2020;133:2803–2807. doi: 10.1097/CM9.0000000000001189
Huan Xia and Yin Wang contributed equally to this work.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.cmj.org).
References
- 1.Yang X, Su B, Zhang X, Liu Y, Wu H, Zhang T. Incomplete immune reconstitution in HIV/AIDS patients on antiretroviral therapy: Challenges of immunological non-responders. J Leukoc Biol 2020; 107:597–612. doi: 10.1002/JLB.4MR1019-189R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marchetti G, Gazzola L, Trabattoni D, Bai F, Ancona G, Ferraris L, et al. Skewed T-cell maturation and function in HIV-infected patients failing CD4+ recovery upon long-term virologically suppressive HAART. AIDS 2010; 24:1455–1460. doi: 10.1097/QAD.0b013e328339cf40. [DOI] [PubMed] [Google Scholar]
- 3.Gaardbo JC, Hartling HJ, Ronit A, Springborg K, Gjerdrum LM, Ralfkiaer E, et al. Regulatory T cells in HIV-infected immunological nonresponders are increased in blood but depleted in lymphoid tissue and predict immunological reconstitution. J Acquir Immune Defic Syndr 2014; 66:349–357. doi: 10.1097/QAI.0000000000000173. [DOI] [PubMed] [Google Scholar]
- 4.Hu W, Wang G, Huang D, Sui M, Xu Y. Cancer immunotherapy based on natural killer cells: current progress and new opportunities. Front Immunol 2019; 10:1205.doi: 10.3389/fimmu.2019.01205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tarazona R, Lopez-Sejas N, Guerrero B, Hassouneh F, Valhondo I, Pera A, et al. Current progress in NK cell biology and NK cell-based cancer immunotherapy. Cancer Immunol Immunother 2020; 69:879–899. doi: 10.1007/s00262-020-02532-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie S, Wu Z, Zhou L, Liang Y, Wang X, Niu L, et al. Iodine-125 seed implantation and allogenic natural killer cell immunotherapy for hepatocellular carcinoma after liver transplantation: a case report. Onco Targets Ther 2018; 11:7345–7352. doi: 10.2147/OTT.S166962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leung W, Iyengar R, Turner V, Lang P, Bader P, Conn P, et al. Determinants of antileukemia effects of allogeneic NK cells. J Immunol 2004; 172:644–650. doi: 10.4049/jimmunol.172.1.644. [DOI] [PubMed] [Google Scholar]
- 8. Cancer Therapy Evaluation Program CTCAE V5.0. USA: National Institutes of Health, 2017. Accessed July 1, 2018 at https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf.). [Google Scholar]
- 9.Coelho L, Cardoso SW, Luz PM, Hoffman RM, Mendonca L, Veloso VG, et al. Vitamin D3 supplementation in HIV infection: effectiveness and associations with antiretroviral therapy. Nutr J 2015; 14:81.doi: 10.1186/s12937-015-0072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards JK, Cole SR, Hall HI, Mathews WC, Moore RD, Mugavero MJ, et al. Virologic suppression and CD4+ cell count recovery after initiation of raltegravir or efavirenz-containing HIV treatment regimens. AIDS 2018; 32:261–266. doi: 10.1097/QAD.0000000000001668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bandera A, Colella E, Rizzardini G, Gori A, Clerici M. Strategies to limit immune-activation in HIV patients. Expert Rev Anti Infect Ther 2017; 15:43–54. doi: 10.1080/14787210.2017.1250624. [DOI] [PubMed] [Google Scholar]
- 12.Shin MH, Kim J, Lim SA, Kim J, Kim SJ, Lee KM. NK cell-based immunotherapies in cancer. Immune Netw 2020; 20:e14.doi: 10.4110/in.2020.20.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helleberg M, Kronborg G, Ullum H, Ryder LP, Obel N, Gerstoft J. Course and clinical significance of CD8+ T-Cell counts in a large cohort of HIV-Infected individuals. J Infect Dis 2015; 211:1726–1734. doi: 10.1093/infdis/jiu669. [DOI] [PubMed] [Google Scholar]
- 14.McBride JA, Striker R. Imbalance in the game of T cells: what can the CD4/CD8 T-cell ratio tell us about HIV and health? PLoS Pathog 2017; 13:e1006624.doi: 10.1371/journal.ppat.1006624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hocqueloux L, Avettand-Fenoel V, Jacquot S, Prazuck T, Legac E, Melard A, et al. Long-term antiretroviral therapy initiated during primary HIV-1 infection is key to achieving both low HIV reservoirs and normal T cell counts. J Antimicrob Chemother 2013; 68:1169–1178. doi: 10.1093/jac/dks533. [DOI] [PubMed] [Google Scholar]


