Supplemental Digital Content is available in the text.
Keywords: arrhythmia, cor pulmonale, coronavirus disease 2019, myocardial ischemia, myocarditis, shock
Abstract
Objectives:
Coronavirus disease 2019 is associated with high mortality rates and multiple organ damage. There is increasing evidence that these patients are at risk for various cardiovascular insults; however, there are currently no guidelines for the diagnosis and management of such cardiovascular complications in patients with coronavirus disease 2019. We share data and recommendations from a multidisciplinary team to highlight our institution’s clinical experiences and guidelines for managing cardiovascular complications of coronavirus disease 2019.
Design, Setting, and Patients:
This was a retrospective cohort study of patients admitted to one of six ICUs dedicated to the care of patients with coronavirus disease 2019 located in three hospitals within one academic medical center in Atlanta, Georgia.
Measurements/Interventions:
Chart review was conducted for sociodemographic, laboratory, and clinical data. Rates of specific cardiovascular complications were assessed, and data were analyzed using a chi-square or Wilcoxon rank-sum test for categorical and continuous variables. Additionally, certain cases are presented to demonstrate the sub committee’s recommendations.
Main Results:
Two-hundred eighty-eight patients were admitted to the ICU with coronavirus disease 2019. Of these, 86 died (29.9%), 242 (84.03%) had troponin elevation, 70 (24.31%) had dysrhythmias, four (1.39%) had ST-elevation myocardial infarction, eight (2.78%) developed cor pulmonale, and 190 (65.97%) with shock. There was increased mortality risk in patients with greater degrees of troponin elevation (p < 0.001) and with the development of arrhythmias (p < 0.001), cor pulmonale (p < 0.001), and shock (p < 0.001).
Conclusions:
While there are guidelines for the diagnosis and management of pulmonary complications of coronavirus disease 2019, there needs to be more information regarding the management of cardiovascular complications as well. These recommendations garnered from the coronavirus disease 2019 cardiology subcommittee from our institution will add to the existing knowledge of these potential cardiovascular insults as well as highlight suggestions for the diagnosis and management of the range of cardiovascular complications of coronavirus disease 2019. Additionally, with the spread of coronavirus disease 2019, our case-based recommendations provide a bedside resource for providers newly caring for patients with coronavirus disease 2019.
The ongoing coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) incurs significant intensive care utilization with approximately 40% of hospitalized patients requiring ICU admission and up to 50% ICU mortality (1–4). The most common complications in critically ill patients are respiratory failure, sepsis, thrombosis, acute kidney injury, heart failure, and cardiac injury (5, 6). Excluding respiratory risk factors, patients most at risk for adverse outcomes include those with underlying cardiovascular disease (CVD) and preexisting CVD, as well as those who develop acute cardiovascular complications from SARS-CoV-2 (7).
The association between COVID illness severity and history of CVD is more marked than previously seen in other viral illnesses, with a 13-fold increased mortality risk, versus 2–9 times increased mortality risk seen with other novel coronavirus infections such as SARS (8–10). Patients with COVID-19 can also suffer devastating cardiovascular injuries from this disease including myocardial infarction, myocarditis, arrhythmias, and hemodynamic instability with cardiogenic shock (11–13).
While there are guidelines on the diagnosis and management of respiratory failure, pneumonia, and sepsis-associated with SARS-CoV-2, there is a lack of guidance for the diagnosis and management of these associated cardiac complications despite the increased prevalence seen in this disease process. The paucity of high-quality best-practice evidence leaves clinicians with equipoise on how to best manage COVID-19 related cardiovascular complications, particularly in the critically ill (9, 11, 12, 14–16). Here, we review our institutional data and describe recommendations detailing the management of acute cardiovascular complications in critically ill patients with COVID-19.
MATERIALS AND METHODS
This study was approved by the Emory University Institutional Review Board. The study included adults greater than or equal to 18 years old with confirmed SARS-CoV-2 disease who were admitted to one of six COVID-designated ICUs at three Emory Healthcare hospitals in Atlanta, Georgia, with hospital admission date from March 6, 2020, to May 6, 2020. Patient data, including sociodemographic information, clinical data, and laboratory data, were obtained from the electronic medical record ([EMR]; Cerner Millennium EMR, Kansas City, MO) and proprietary data collection software developed on the Oracle Apex platform (Redwood City, CA).
Cardiac complications included troponin elevation, arrhythmia captured on electrocardiogram, ST-elevation myocardial infarction (STEMI) on electrocardiogram, myocarditis confirmed by tissue pathology, cor pulmonale by clinical diagnosis and/or echocardiogram, shock by clinical diagnosis and/or vasopressor requirement for 2 hours or more, cardiac arrest with cardiopulmonary resuscitation (CPR) performed.
Codified and continuous data elements were automatically extracted using an internally validated technique from the EMR. Data elements contained in free-text were manually abstracted by the members of the Emory COVID-19 Quality and Clinical Research Collaborative and entered into the Apex platform (17). Data were analyzed using a chi-square or Wilcoxon rank-sum test for categorical and continuous variables, respectively. A p value of less than 0.05 was defined as significant. All tests were two-sided and were performed using SAS software, Version 9.4 (SAS Institute, Cary, NC).
To create immediate and dynamic guidelines for the care of critically ill patients with COVID-19, our institution implemented a team best practice committee with organ-specific subcommittees (17). The cardiology subcommittee was comprised of fellow and attending physicians from both cardiology and critical care. The cardiology members’ subspecialties included interventional, electrophysiology, advanced heart failure, and critical care. The critical care members’ specialties included emergency medicine, anesthesiology, and internal medicine.
The cardiology subcommittee met weekly to review cases and collectively create situational specific recommendations to be shared with frontline providers across the healthcare network (17). The guidelines were rapidly adjusted in overlapping stages which incorporated feedback from frontline clinicians, up-to-date literature review, drafting the first checklist version involving an internal expert panel, roundtable panel discussions, and finalization of the algorithms and recommendations after panel agreement. We present representative cases discussed by the committee which laid the foundation for the development of institutional practice guidelines and recommendations.
RESULTS
Out of 288 patients diagnosed and admitted to the ICU with COVID-19, 86 died (29.9%) (Table 1) and 258 (89.6%) incurred diagnoses relevant to critical care cardiology (Table 2).
TABLE 1.
Demographic Characteristics of Patients Admitted to the ICU With Coronavirus Disease 2019
| Characteristic (n [%] Unless Otherwise Indicated) | Total (n = 288) | Survived Hospitalization (n = 202) | Died During Hospitalization (n = 86) | p a |
|---|---|---|---|---|
| Age, median (IQR) | 65 (55–75) | 61 (51–69) | 73 (65–80) | < 0.001 |
| Female | 131 (45.5) | 93 (40.1) | 38 (44.2) | 0.773 |
| Race | 0.778 | |||
| White | 54 (18.8) | 40 (18.8) | 14 (16.3) | |
| Black | 198 (68.8) | 138 (68.3) | 60 (69.8) | |
| Asian | 9 (3.1) | 5 (2.5) | 4 (4.7) | |
| Unknown/other | 27 (9.4) | 19 (9.4) | 8 (9.3) | |
| Body mass index, median (IQR) | 30.3 (25.9–35.3) | 31.1 (27.2–37.2) | 28.5 (24.8–31.3) | < 0.001 |
| ≥ 40 | 35 (12.2) | 34 (16.8) | 1 (1.2) | |
| Tobacco use | 81 (28.1) | 49 (24.3) | 32 (37.2) | 0.025 |
| Hypertension | 214 (79.0) | 144 (77.4) | 70 (82.4) | 0.072 |
| Coronary artery disease | 40 (17.8) | 22 (13.8) | 18 (27.3) | 0.024 |
| Congestive heart failure | 39 (16.9) | 28 (17.4) | 11 (15.7) | 0.808 |
| Diabetes mellitus | 129 (44.8) | 90 (44.6) | 39 (45.3) | 0.901 |
| Chronic kidney disease/end-stage renal diseaseb | 43 (14.9) | 26 (12.9) | 17 (19.8) | 0.305 |
| Asthma | 28 (11.5) | 23 (13.5) | 5 (6.8) | 0.129 |
| Chronic obstructive pulmonary disease | 19 (7.9) | 11 (6.6) | 8 (10.5) | 0.228 |
| Obstructive sleep apnea | 24 (10.5) | 20 (12.4) | 4 (6.0) | 0.140 |
| ACE inhibitor or ARB exposure | 97 (33.7) | 61 (30.2) | 36 (41.9) | 0.055 |
| ACE inhibitor | 47 (16.3) | 33 (16.3) | 14 (16.3) | 0.990 |
| ARB | 54 (18.8) | 31 (15.3) | 23 (26.7) | 0.023 |
| Statin | 105 (36.5) | 72 (35.6) | 33 (38.4) | 0.669 |
ACE = angiotensin-converting enzyme, ARB = angiotensin II receptor blocker, IQR = interquartile range.
aχ2 or Wilcoxon rank-sum test.
bChronic kidney disease stage 3 or higher or end-stage renal disease requiring dialysis.
TABLE 2.
Cardiovascular Sequelae in Patients Admitted to the ICU With Coronavirus Disease 2019
| Characteristic (n [%] Unless Otherwise Indicated) | Total (n = 288) | Survived Hospitalization (n = 202) | Died During Hospitalization (n = 86) | p a |
|---|---|---|---|---|
| Cardiac complication | 258 (89.6) | 173 (85.6) | 85 (98.8) | < 0.001 |
| Troponin elevation | 242 (84.0) | 160 (79.2) | 82 (95.4) | < 0.001 |
| Median (interquartile range) | 0.11 (0.05–0.42) | 0.09 (0.04–0.23) | 0.36 (0.08–1.29) | < 0.001 |
| > 0.4 (99%) | 21 (7.6) | 9 (4.6) | 12 (14.8) | 0.004 |
| Arrhythmia | 70 (24.3) | 32 (15.8) | 38 (44.2) | < 0.001 |
| ST-elevation myocardial infarction | 4 (1.4) | 2 (1.0) | 2 (2.3) | 0.375 |
| Myocarditisb | ||||
| Cor pulmonale | 8 (2.8) | 1 (0.5) | 7 (8.1) | < 0.001 |
| Shock | 190 (66.0) | 120 (59.4) | 70 (81.4) | < 0.001 |
| Cardiac arrest with cardiopulmonary resuscitation | 32 (11.1) | 6 (3.0) | 26 (30.2) | < 0.001 |
aχ2 or Wilcoxon rank-sum test.
bUnable to measure with no pathology confirmed cases at time of this report.
The median age of the critically ill COVID-19 patients was 65 years (interquartile range, 55–75 yr). Hypertension (214/288 [79.0%]) and diabetes (129/288 [44.8%]) were the most common preexisting conditions (Table 1). There was a statistically significant difference in mortality rates in patients with preexisting coronary artery disease as well as patients prescribed an angiotensin II receptor blocker (ARB) as an outpatient medication. Of the patients with COVID, 13.8% who survived had coronary artery disease (CAD) compared with 27.3% of those who died during their hospitalization that had CAD (p = 0.024). Of the patients admitted to the ICU for COVID, 15.3% who survived were on ARB as an outpatient medication versus to 26.7% who died (p = 0.023). Conversely, there was no difference in mortality rates among patients with COVID who were prescribed an angiotensin-converting enzyme (ACE) inhibitor as an outpatient medication (13.3% survived, 18.7% died; p = 0.990).
Laboratory markers associated with statistically significant increased mortality rates include elevation in troponin, B-type natriuretic peptide (p < 0.001), d-dimer (p < 0.001), C-reactive protein (CRP) (p < 0.001), and lactic acid (p = 0.043) (Supplemental Material - Table 3, http://links.lww.com/CCX/A431).
The most common cardiovascular complications in patients with COVID-19 were troponin elevation, arrhythmias, cor pulmonale and shock, and the development of these was associated with a statistically significant increased risk for mortality (Table 2). Below, we also present select cases that highlighted and helped refine our guidelines (Fig. 1) selected by the members of the cardiology subcommittee.
Figure 1.

Coronavirus Disease 2019 (COVID-19) Cardiac Guideline Recommendations phone card reference. ATIII = antithrombin III, C3/C4 = complement levels 3 and 4, CCB = calcium channel blocker, CRP = C-reactive protein, DHP = dihydropyridine, ECG = electrocardiogram, ECHO = echocardiography, ECMO = extracorporeal membrane oxygenation, ESR = erythrocyte sedimentation rate, HF = heart failure, H&P = history and physical, IABP = intra-aortic balloon pump, IJ = internal jugular, IL-6 = interleukin 6, LDH = lactate dehydrogenase, LV = left ventricle, LVAD = left ventricular assist device, PEEP = positive end-expiratory pressure, POCUS = point of care ultrasound, RV = right ventricle, STEMI = ST-elevation myocardial infarction, PCI = percutaneous coronary intervention, QT = QT interval, QTc = corrected QT interval, SvO2 = mixed venous oxygen saturation.
CASES AND DISCUSSION
Several studies identified that markers of inflammation and myocardial injury, like troponin T elevation, are associated with poor outcomes and overall increased mortality rates (9, 11, 12, 14, 16, 18). Our data also showed that troponin elevation, particularly as levels continue to rise, is associated with increased mortality rates. While associated with worse outcomes, the mechanism driving troponin elevation in these patients is unclear.
Hypoxia, a common presentation of COVID-19 pneumonia, can drive myocardial oxygen supply and demand mismatch. An underlying cardio-inflammatory response, indicated by elevated levels of CRP, ferritin, and other acute phase reactants, may also lead to troponin elevation. Coronavirus has localized to myocardial interstitial cells, and focal cardiomyocyte necrosis and lymphocytic myocarditis have been demonstrated in autopsy or by myocardial biopsy of these patients. Alternative etiologies for myocardial injury and release of troponin during SARS-CoV-2 infection are right ventricular (RV) overload, pulmonary embolism, cytokine-mediated injury, microvascular dysfunction, small vessel thrombotic complications, epicardial coronary obstruction or thrombosis, pericarditis, or stress-induced cardiomyopathy. Troponin elevations in COVID-19 patients are thus varied and likely multifactorial.
With multiple potential underlying etiologies, troponin elevation is commonly incurred and may be seen in up to 20% of patients with COVID-19 (4, 19). As troponin elevation can be commonly seen in this patient population, particularly those who are critically ill and require ICU admission, we will now present several cases highlighting how troponin elevation can be used in conjunction with other data to help determine the likely etiology of myocardial injury and clinical implications for management.
Troponin Elevation With Electrocardiogram Changes
Case.
Sixty-eight-year-old female was admitted for hypoxic respiratory failure requiring intubation from COVID pneumonia. While intubated, she developed deeply inverted T waves across the precordial leads on electrocardiogram (Supplemental Figure, http://links.lww.com/CCX/A432), and troponin I peaked at 3.83 ng/mL. A transthoracic echocardiogram (TTE) demonstrated a reduced left ventricular ejection fraction (LVEF) of 35%, apical akinesis, and a layered apical thrombus. She remained hemodynamically stable and was extubated 18 days after intubation; however, her electrocardiogram continued to illustrate deep T wave inversions even after troponin levels declined, which was most likely secondary to known apical akinesis (Fig. 2). Given the overall presentation, the most likely diagnosis was a stress-induced cardiomyopathy for which systemic anticoagulation, for the apical thrombus, and dual antiplatelet therapy, in the abundance of caution without coronary angiogram data, were initiated.
Figure 2.
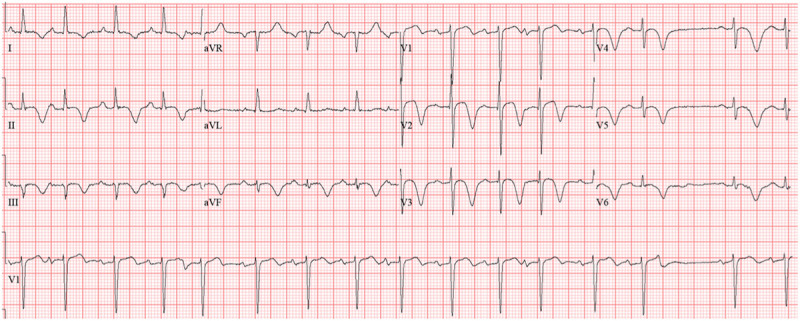
Electrocardiogram 2 wk later with ongoing T wave inversion in the precordial leads. aVF = augmented vector foot, aVL = augmented vector left, aVR = augmented vector right.
Discussion.
Following intubation, the patient’s troponin level increased approximately 10-fold over a 24-hour period. The change in troponin levels coincided with electrocardiogram changes suggestive of myocardial ischemia and injury. In this patient, troponin elevation and electrocardiogram changes were associated with echocardiographic findings characteristic of stress-induced cardiomyopathy with apical ballooning. Peak troponin levels in stress-induced cardiomyopathy are typically less than one would see in a true myocardial infarction, and, in this patient, peak troponin levels were less than would be expected for apical dysfunction caused by left anterior descending artery occlusion (19). After peaking at 3.83 ng/mL, troponin levels returned to baseline over the next several days as her respiratory status stabilized.
The current COVID pandemic has presented many unique challenges in the invasive management of STEMI and non-STEMI. The differential diagnosis of dynamic ST changes has been greatly widened in this population. Not all ST elevation represents typical type 1 STEMI with plaque rupture where early revascularization may be beneficial and may instead indicate hemodynamic stress, direct inflammatory myocarditis or pericarditis, stress cardiomyopathy, or microvascular disease due to thrombosis or hyperviscosity. The decision to activate the Cardiac Catheterization Laboratory has to take into consideration pretest probability for detection of hemodynamically significant epicardial coronary stenoses, as well as the potential benefits versus harms for revascularization and need to minimize exposure to medical personnel (20). Our experience at Emory Healthcare has shown that the occurrence rate of type 1 STEMI has been much lower than expected. This has been collaborated by reports from other institutions where the occurrence rate of STEMI activations was markedly decreased during the height of the COVID pandemic (21).
Troponin Elevation With Echocardiographic Changes
Pericarditis Case.
Sixty-one-year-old female was admitted for hypoxic respiratory failure from COVID-19 requiring intubation and ultimately venovenous extracorporeal membrane oxygenation (ECMO). Initial electrocardiogram was unremarkable; however, 10 days following admission, telemetry changes prompted another formal electrocardiogram that demonstrated diffuse ST elevations (Fig. 3). Troponin I was only mildly elevated and peaked at 0.05 ng/mL. TTE showed preserved LVEF of 60%, no regional wall motion abnormalities, and a small pericardial effusion. Given the diffuse concave ST elevations, the normal echocardiogram, and low-level troponin elevation with acute rise and fall, this presentation was consistent with pericarditis. ST changes eventually resolved, and she was discharged to a long-term acute care facility on hospital day 39.
Figure 3.
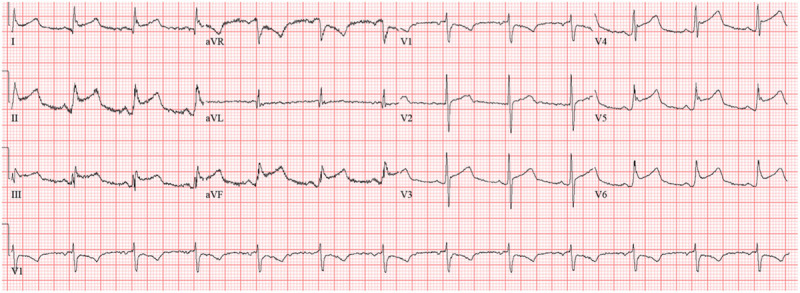
Electrocardiogram demonstrating diffuse ST elevation. aVF = augmented vector foot, aVL = augmented vector left, aVR = augmented vector right.
Myocarditis Case.
Twenty-four-year-old female was admitted with hypoxic respiratory failure from COVID pneumonia and required intubation on hospital day 2. Later that evening, she suffered ventricular fibrillation with cardiac arrest. Following return of spontaneous circulation (ROSC), TTE demonstrated new onset biventricular failure with a small pericardial effusion. She continued to require increasing doses of vasopressors and inotropes and was subsequently cannulated for venoarterial ECMO given concern for myocarditis. Unfortunately, the patient did not have neurologic recovery following decannulation from ECMO, and brain MRI demonstrated diffuse anoxic brain injury. The decision was made to terminate life-sustaining treatments at that time, and she passed away.
Discussion.
COVID-19 is associated with high degrees of inflammation affecting multiple organ systems. As part of this invasive inflammatory process, patients may develop severe systemic cytokine-mediated myocarditis that may be associated with arrhythmias, cardiomyopathy, or epicardial or microvascular thrombosis. Limited data exist on the prevalence of inflammatory infiltration in this patient population (22). As of this writing, there are only two cases in the published literature demonstrating a lymphocytic infiltrate or evidence of myocardial injury (23, 24).
There is a proposed mechanism to how this virus may cause myocyte damage. S-glycoprotein on the surface of SARS-CoV-2 binds to ACE2 and causes a conformational change in the S-glycoprotein. This allows proteolytic digestion by host cell proteases (transmembrane serine protease 2 and furin), ultimately leading to internalization of the virus (25). SARS-CoV-2’s entry through ACE2 results in an inflammatory response but also subsequently downregulates ACE2 expression, resulting in an inability to protect against the inflammatory response (25, 26). Furthermore, cellular entry of the virus triggers an inflammatory response with recruitment of T-helper cells which produce interferon gamma. This leads to recruitment of other inflammatory cells and a “cytokine storm,” which could lead to organ damage and multiple organ failure seen in severe disease.
Down-regulation of ACE2 activity may result in unopposed angiotensin II accumulation and local renin-angiotensin-aldosterone system (RAAS) activation. The resultant unopposed angiotensin II accumulation and local RAAS activation contribute to end-organ injury and likely cardiovascular damage. We have significant concerns that the long-term effects of infection will lead to chronic progressive cardiomyopathy, arrhythmias, or vascular disease.
Troponin Elevation With Cardiac Arrhythmias
Case.
Sixty-nine-year-old male was admitted for hypoxic respiratory failure secondary to COVID-19. Initial electrocardiogram demonstrated normal sinus rhythm with first-degree atrioventricular block and a nonspecific interventricular conduction delay (QRS complex [QRS] 128 msec). The patient’s condition declined, requiring intubation, and over the next 48 hours, he developed progressively worsening electrocardiogram findings, now with first-degree atrioventricular block, corrected QT interval (QTc) prolongation, and worsening interventricular conduction delay. On the fourth hospital day, the patient’s conduction disease progressed to complete heart block and then to complete heart block with a wide QRS. The patient remained in this rhythm until he died after his family elected to pursue comfort care measures.
Discussion.
Arrhythmias are a well-described complication of COVID-19, although little data are available to elucidate the exact pathophysiology of this phenomenon (3, 27). Our data showed that 24.3% of patients admitted to the ICU with COVID-19 developed arrhythmias. Other case series have reported an occurrence rate of arrhythmias of 16.7%, with a greater likelihood for malignant arrhythmias in particular when evidence of myocardial injury is present (3, 14). One analysis, however, showed that only 5.9% of in-hospital cardiac arrests in patients with COVID-19 were from shockable rhythms, which compares to the American Heart Association statistics showing 24% of all patients with in-hospital cardiac arrests had a shockable rhythm (22, 28).
It also remains unclear what proportion of the observed arrhythmias are related to medication use including medications notorious for prolonging QTc such as hydroxychloroquine and azithromycin. While some medications implicated in arrhythmias are of unclear benefit to patients and are no longer routinely being used, early use of these medications has made it difficult to retrospectively study early presentations of SARS-CoV-2 infection.
It is also important to address any electrolyte abnormalities and aggressively replete electrolytes as needed. Patients with COVID-19 may be at risk for hypokalemia as SARS-CoV-2 binds to ACE2 receptors, increasing angiotensin II and thus potentially increasing urinary potassium excretion (29). Aggressive electrolyte protocols have been implemented at our institution, including maintaining a potassium greater than 4.5 mmol/L and magnesium level greater than 2.5 mg/dL.
Troponin Elevation With Cor Pulmonale
Case.
Forty-two-year-old male was admitted with hypoxic respiratory failure secondary to COVID-19, requiring intubation on hospital day 2. He was diagnosed with a new extensive lower extremity deep venous thrombosis on hospital day 5, for which he was anticoagulated. After substantial ventilator weaning to minimal settings, he suffered a cardiac arrest on hospital day 9. Presumed due to massive pulmonary embolism with bedside ultrasound with newly dilated RV, he received two doses of 50 mg tissue plasminogen activator (tPA) during the code with ROSC obtained after roughly 50 minutes of CPR. Several days later as vasopressors were weaned, ultrasound revealed improvement in RV size and function, and he was able to be extubated 2 days later on hospital day 11. Computed tomography angiography on hospital day 12 revealed large pulmonary emboli in the left main pulmonary artery and left upper lobe lobar branches. Patient was ultimately discharged home on room air on hospital day 18.
Discussion.
While the disease pathophysiology is still under investigation, COVID-19 is associated with coagulopathy with high rates of thrombotic events that are linked to higher morbidity and mortality (27, 30, 31). Similar to our case presented here, there have been multiple documented cases of cardiac arrest secondary to acute cor pulmonale from massive pulmonary embolism in patients with COVID-19 which have occurred along any point in a patient’s hospitalization, including while on therapeutic anticoagulation (32). As such, patients should receive systemic tPA even while on therapeutic anticoagulation if they develop acute hemodynamic disability or suffer cardiac arrest with a concomitant abrupt increase in RV size or decrease in RV function. Additionally, embolectomy or ECMO cannulation is considered, and for patients who are not considered an ECMO candidate, should receive supportive care for RV failure, including ongoing anticoagulation, inhaled epoprostenol or nitric oxide, and dobutamine for RV inotropic support.
Troponin Elevation With Hemodynamic Instability and Shock
Case.
Sixty-six-year-old male was admitted for hypoxic respiratory failure from COVID-19 and required intubation on hospital day 2. He had a long hospital course that was complicated by septic shock secondary to methicillin-susceptible Staphylococcus aureus bacteremia and Clostridium difficile infection. Echocardiogram demonstrated an ejection fraction of 55%. Despite antibiotics and line removal for attempted source control, he required maximum dosage of multiple vasopressors, including 3 mcg/kg/min norepinephrine, 0.03 U/min vasopressin, 5 mcg/kg/min phenylephrine, and eventually died secondary to refractory vasoplegic shock.
Discussion.
This case demonstrates elements of hemodynamic complications associated with COVID-19. Shock has been frequently seen, with our data showing that 65.7% of patients required vasopressor support, and other studies showing similar rates with nearly 70% of patients requiring vasopressors or inotropes (4, 15). The Global Sepsis Alliance concluded that COVID-19 induces sepsis physiology and therefore is capable of resulting in septic shock (4, 15). COVID-19 itself is not typically associated with severe degrees of shock requiring higher doses of vasopressors. Rather, the requirement for higher doses of vasopressors or inotropes should prompt suspicion for other processes such as a coinfection or superinfection leading to septic shock or underlying obstructive or cardiogenic shock from massive pulmonary embolus, myocarditis, or Takotsubo cardiomyopathy.
CONCLUSIONS
The cardiovascular sequelae of COVID-19 can be profound and impact mortality. While there are guidelines for the diagnosis and management of pulmonary complications of COVID-19, there is a dearth of evidence regarding the management of cardiovascular complications of COVID-19. Our data has shown that underlying CVD such as CAD place patients at increased risk for mortality. In addition, laboratory markers such as troponin elevation, in addition to the development of shock and cardiac arrhythmias, can portend poorer prognoses and should prompt particular attention to specific cardiovascular management. With the continued spread of COVID-19, our recommendations specifically provide educational case-based guidance with a bedside reference resource (Fig. 1) for providers newly caring for patients with cardiac complications and COVID-19.
ACKNOWLEDGMENTS
Emory COVID-19 Quality and Clinical Research Collaborative Members (in alphabetical order): Max W. Adelman, Scott Arno, Sara C. Auld, Theresa Barnes, William Bender, James M. Blum, Gaurav Budhrani, Stephanie Busby, Laurence Busse, Mark Caridi-Scheible, David Carpenter, Nikulkumar Chaudhari, Craig M. Coopersmith, Lisa Daniels, Johnathan A. Edwards, Jane Fazio, Babar Fiza, Eliana Gonzalez, Ria Gripaldo, Charles Grodzin, Robert Groff, Alfonso C. Hernandez-Romieu, Max Hockstein, Dan Hunt, Craig S. Jabaley, Jesse T. Jacob, Colleen Kraft, Greg S. Martin, Samer Melham, Nirja Mehta, Chelsea Modlin, David J. Murphy, Jung Park, Deepa Patel, Cindy Powell, Amit Prabhakar, Jeeyon Rim, Ramzy Rimawi, Chad Robichaux, Nicholas Scanlon, Milad Sharifpour, Bashar Staitieh, Michael Sterling, Jonathan Suarez, Colin Swenson, Nancy Thakkar, Alexander Truong, Hima Veeramachaneni, Alvaro Velasquez, Michael Waldmann, Max Weinmann, Thanushi Wynn, and Joel Zivot.
Supplementary Material
Footnotes
Medical illustrator: Caroline Coleman, MD.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccxjournal).
The authors have disclosed that they do not have any potential conflicts of interest.
Contributor Information
Collaborators: Max W. Adelman, Scott Arno, Sara C. Auld, Theresa Barnes, William Bender, James M. Blum, Gaurav Budhrani, Stephanie Busby, Laurence Busse, Mark Caridi-Scheible, David Carpenter, Nikulkumar Chaudhari, Craig M. Coopersmith, Lisa Daniels, Johnathan A. Edwards, Jane Fazio, Babar Fiza, Eliana Gonzalez, Ria Gripaldo, Charles Grodzin, Robert Groff, Alfonso C. Hernandez-Romieu, Max Hockstein, Dan Hunt, Craig S. Jabaley, Jesse T. Jacob, Colleen Kraft, Greg S. Martin, Samer Melham, Nirja Mehta, Chelsea Modlin, David J. Murphy, Jung Park, Deepa Patel, Cindy Powell, Amit Prabhakar, Jeeyon Rim, Ramzy Rimawi, Chad Robichaux, Nicholas Scanlon, Milad Sharifpour, Bashar Staitieh, Michael Sterling, Jonathan Suarez, Colin Swenson, Nancy Thakkar, Alexander Truong, Hima Veeramachaneni, Alvaro Velasquez, Michael Waldmann, Max Weinmann, Thanushi Wynn, and Joel Zivot
REFERENCES
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China Lancet 2020; 395:497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gold JA, Wong K, Szablewski CM, et al. Characteristics and clinical outcomes of adult patients hospitalized with COVID-19 — Georgia, March 2020 MMWR Morb Mortal Wkly Rep 2020; 69:545–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China JAMA 2020; 323:1061–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle region - case series. N Engl J Med 2020; 382:2012–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study BMJ 2020; 368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bilaloglu S, Aphinyanaphongs Y, Jones S, et al. Thrombosis in hospitalized patients with COVID-19 in a New York City Health System. JAMA 2020; 324:799–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zaim S, Chong JH, Sankaranarayanan V, et al. COVID-19 and multiorgan response. Curr Probl Cardiol 2020; 45:100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang Y, Chen T, Mui D, et al. Cardiovascular manifestations and treatment considerations in Covid-19 Heart 2020; 106:1132–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hessami A, Shamshirian A, Heydari K, et al. Cardiovascular diseases burden in COVID-19: Systematic review and meta-analysis. Am J Emerg Med 2020. Oct 16. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention JAMA 2020; 23:1239–1242 [DOI] [PubMed] [Google Scholar]
- 11.Driggin E, Madhavan MV, Bikdeli B, et al. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID-19) pandemic J Am Coll Cardiol 2020; 75:2352–2371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Long B, Brady WJ, Koyfman A, et al. Cardiovascular complications in COVID-19 Am J Emerg Med 2020; 38:1504–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inciardi RM, Lupi L, Zaccone G, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol 2020; 5:819–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol 2020; 5:811–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA 2020; 323:1612–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). 2020. Available at: https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf. Accessed May 15, 2020
- 17.Emory Healthcare. Safety Center & COVID-19 Resources. 2020. Available at: https://www.emoryhealthcare.org/covid/medical-professionals.html. Accessed June 1, 2020
- 18.Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China JAMA Cardiol 2020; 5:802–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020; 395:1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Welt FGP, Shah PB, Aronow HD, et al. American College of Cardiology’s Interventional Council and the Society for Cardiovascular Angiography and Interventions. Catheterization laboratory considerations during the coronavirus (COVID-19) pandemic: From the ACC’s Interventional Council and SCAI. J Am Coll Cardiol 2020; 75:2372–2375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia S, Albaghdadi MS, Meraj PM, et al. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the United States during COVID-19 pandemic. J Am Coll Cardiol 2020; 75:2871–2872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adunsky A, Blumen N, Ohry A. [Stroke in the young]. Harefuah 1990; 119:311–312 [PubMed] [Google Scholar]
- 23.Sala S, Peretto G, Gramegna M, et al. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J 2020; 41:1861–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tavazzi G, Pellegrini C, Maurelli M, et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail 2020; 22:911–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor Cell 2020; 181:271–280.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 2005; 11:875–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy J Thromb Haemost 2020; 18:1094–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meaney PA, Nadkarni VM, Kern KB, et al. Rhythms and outcomes of adult in-hospital cardiac arrest. Crit Care Med 2010; 38:101–108 [DOI] [PubMed] [Google Scholar]
- 29.Chen D, Li X, Song Q, et al. Hypokalemia and clinical implications in patients with coronavirus disease 2019 (COVID-19) medRxiv 2020; 3:e2011122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020; 191:145–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levi M, Thachil J, Iba T, et al. Coagulation abnormalities and thrombosis in patients with COVID-19 Lancet Haematol 2020; 7:e438–e440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Creel-Bulos C, Hockstein M, Amin N, et al. Acute cor pulmonale in critically ill patients with Covid-19. N Engl J Med 2020; 382:e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


