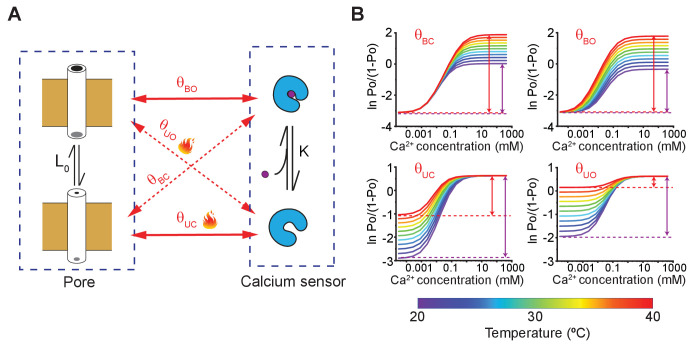
Figure 5. A simple allosteric model for temperature activation of MthK.
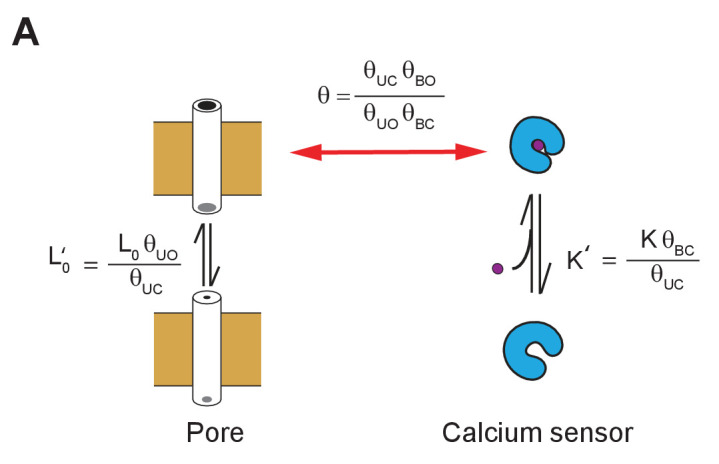
(A) Four state binary elements model of MthK activation. The intrinsic equilibrium constant of pore opening is L0 and the calcium-binding affinity is K. The state-dependent interactions between the pore and calcium sensor are represented by , where X indicates the conformation of the calcium sensor (X = bound (B) or unbound (U)) and Y indicates the conformation of the Pore (Y = open (O) or closed (C)). Solid lines indicate the interactions between 'like' states and the dotted lines highlight interactions between 'unlike' states. (B) Using the model of MthK channel gating, described in A, we simulated the Hill-plot of MthK (i.e. ln [Po/(1-Po)] vs. Ca2+) at different temperatures for various model parameters. In each graph, all parameters, except the state-dependent interaction terms shown in each sub-panel, were kept constant across different temperatures. For all simulations, the values of the parameters used were: L0 = 0.1; K = 20000 M−1; 8; 18; 150; 8. For temperature-dependent simulations of , we use the following equation: , where -71 kJ and -220 J/K. Similarly, ( 81 kJ and 310 J/K), ( -71 kJ and -220 J/K) and ( 80 kJ and 300 J/K) were calculated.
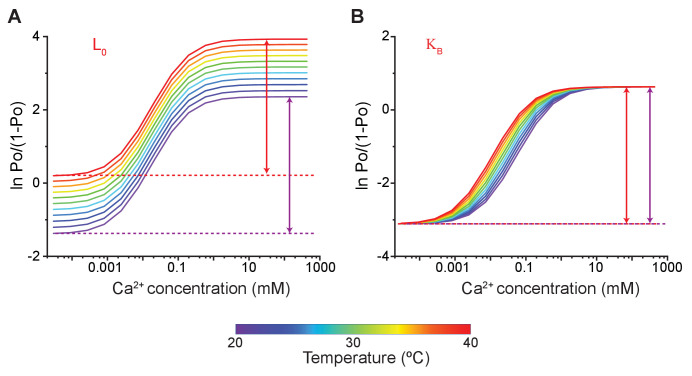
Figure 5—figure supplement 1. Effect of temperature-dependent parameters on simulated Hill-plots of calcium-dependent gating of MthK.
Figure 5—figure supplement 2. MthK activation involving multiple calcium binding sites with differing temperature-dependence.
Figure 5—figure supplement 3. An alternate allosteric model of MthK activation involving independent calcium and temperature sensing domains.
Figure 5—figure supplement 4. Renormalization of state-dependent interaction parameters.