Abstract
The ongoing coronavirus disease 2019 (COVID-19) pandemic is a global public health crisis, causing social and economic disasters in many countries. In China, two-consecutive negative results of nucleic acid tests for SARS-CoV-2 from the respiratory samples are required to end the quarantine of COVID-19 patients. However, clinicians face a dilemma in case of patients with long-term viral shedding. This report described an unusual COVID-19 case who had persistent viral RNA positivity for more than 4 months after initial illness in the presence of low neutralizing antibodies, but without prolonged clinical symptoms. Multiple anti-viral drug treatments had no impact and there was no evidence of re-infection. When the patient was self-quarantined at home, no infection occurred to the three family members living with her for 15 to 19 days. Sputum viral culture in BSL-3 laboratory on the 102nd day after symptom onset was negative. From the 129th day on, 8 continuous nucleic acid tests of sputum samples showed negative results. The patient was discharged on 137th days since symptom onset. In conclusion, viral RNA shedding in the sputum of the COVID-19 patient may last over 4 months. As no evidence shows the existence of infectious virus, two-consecutive negative nucleic acid tests may not be the prerequisite for ending quarantine of COVID-19 patients with prolonged viral shedding.
Keywords: COVID-19, SARS-CoV-2, viral shedding, neutralizing antibodies, quarantine
Introduction
The coronavirus disease 2019 (COVID-19) pandemic is a huge threat to global public health. As the first country to confront this novel virus, China has sequentially developed seven versions of interim clinical guidance for diagnosis and treatment of COVID-19[1]. Stringent contact tracing and isolation strategy has also been applied in China. All COVID-19 cases, regardless of symptoms, are isolated and treated in local hospitals until obtaining negative results in two-consecutive nucleic acid tests for SARS-CoV-2 from the respiratory samples[1]. Here, we reported an unusual COVID-19 case with continuing viral shedding after recovery of COVID-19. On May 24, which was over 4 months after initial illness onset, the patient was still positive for SARS-CoV-2. Clinical challenge related to this special condition was discussed.
The case report was approved by the ethics committee of the Second Hospital of Nanjing (ethics approval No.: 2020-LS-ky003).
Case report
A 68-year-old female was admitted to our hospital on January 21, 2020, after suffering from productive cough and sore throat for 4 days. She was previously healthy without any underlying comorbidity and had stayed in Wuhan for 15 days before returning to Nanjing on January 16, 2020. At the time of admission, physical examination showed unremarkable findings. Routine blood indexes, including complete blood count, liver and renal function, creatine kinase, lactate dehydrogenase, myocardial enzymes, coagulation profile, interleukin-6, procalcitonin, C-reactive protein, and erythrocyte sedimentation rate were within the normal range. Serum IgM antibodies to influenza A and B viruses, and parainfluenza virus were all negative, and serum HIV antibody was non-reactive. A chest computed tomography (CT) scan showed small ground-glass opacity (GGO) in the right lower lobe of the lung. SARS-CoV-2 infection was confirmed on the same day by real-time PCR from throat swab sample using previously described method[2]. Since then, the relative viral loads of SARS-CoV-2 were monitored almost every other day (Fig. 1A).
1.
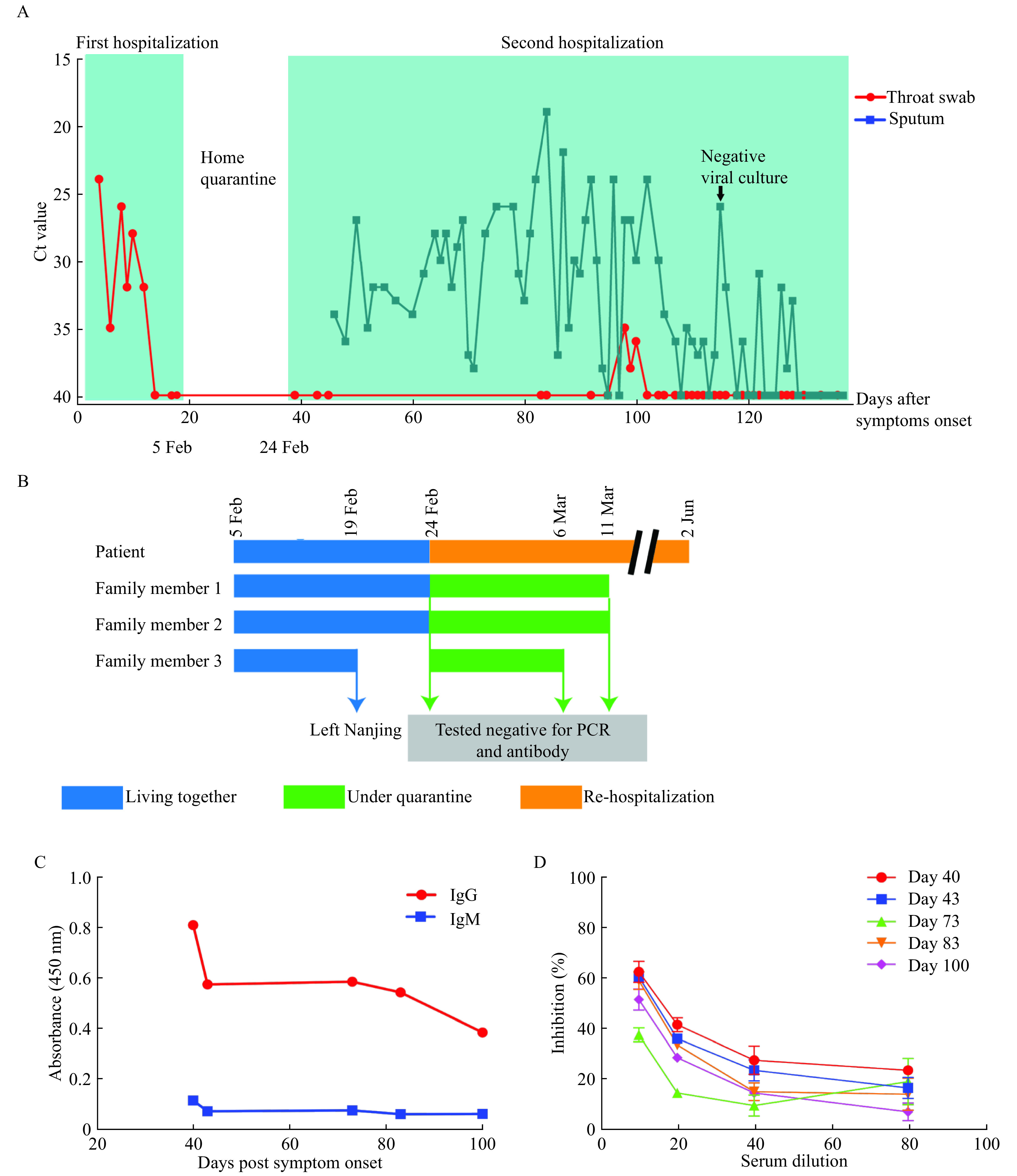
Summary of key dates, events, and data.
A: Timeline summary of the two hospitalization events together with data of viral RNA detection. B: Contact tracing of family members and other results for viral RNA and antibodies. C and D: Results for antibody tests conducted on day 40, 43, 73, 83, and 100 post symptom onsets for IgM/IgG (C) and neutralizing antibodies (D). Testing for IgM and IgG was conducted at a serum dilution of 1:80 using ELISA kits from GenScript following the manufacturer's instructions.
The patient was given combined antiviral therapy with interferon-α (5 000 000 U, twice a day, aerosol inhalation) from January 22 to February 5 and lopinavir/ritonavir (400/100 mg, twice a day) from January 23 to February 5. In addition, she received intravenous immunoglobulin treatment (20 g per day) for 5 days from January 23 to January 27. But her clinical condition was not improved. She developed fever on January 25 and displayed progression of pneumonia involving all five lung lobes on January 27 as shown by radiological evidence. A 3-day course of intravenous methylprednisolone (40 mg per day) was then administered starting from January 28, which substantially improved the patient's clinical signs, including pneumonia. The patient was discharged after three consecutive negative PCR results on February 5, 19 days after symptom onset (Fig. 1A).
After discharge, the patient was advised self-quarantine at home, living with three other family members in the same household (Fig. 1B). On February 22, the local CDC staff visited the patient to take throat swab sample for retest. As the result was inconclusive, an induced sputum sample was taken on February 23 and was tested PCR positive, which was 37 days after symptom onset. Despite the absence of clinical symptoms and normal CT scan results, the patient was hospitalized again on February 24 following the local CDC policy that patients with positive nucleic test result for SARS-CoV-2 are required to be monitored in the hospital. Since then, both throat swab and induced sputum samples were taken for PCR testing. During the second hospitalization, the patient was treated with aerosol interferon-α (5 000 000 U, twice a day) continuously. She was also sequentially treated with arbidol (0.2 g, every 8 hours) for 2 weeks and chloroquine phosphate (0.5 g, twice a day) for 1 week. Though without any clinical symptoms or apparent lung lesion on the chest CT during the second hospitalization, SARS-COV-2 viral RNA was persistently detected in her sputum samples. On April 28, 2020, 102 days after symptom onset, her sputum sample taken for viral culture by local CDC was tested negative. As of May 24, which was over 4 months after symptom onset, her sputum was still positive for SARS-CoV-2 (Fig. 1A).
An immunological investigation of the patient was conducted. At the time of the first admission, the lymphocyte count was within the normal range (800 to 4000 cells/L). Lymphocyte subgroup analysis indicated low CD8+ T lymphocyte count (216 cells/µL) and low percentage (17%) (Table 1), which persisted during the disease course. In addition, the lymphocyte counts substantially decreased and then gradually returned to normal as the clinical condition improved. On the 83rd day post symptom onset, a multiplex cytokine analysis was performed. Plasma levels of IL-2, IL-4, IL-6, IL-10, TNF-a, IFN-r, and IL-17A were all within the reference range.
1. Lymphocyte subsets counts at different time points post symptom onset.
| Time from illness
onset to sample collection (days) |
Lymphocyte
counts (cells/μL) |
CD3+
(cells/μL) (%) |
CD3+CD4+
(cells/μL) (%) |
CD3+CD8+
(cells/μL) (%) |
CD4+/CD8+ ratio |
CD16+/CD56+NK
cells (cells/μL) (%) |
CD19+
(cells/μL) (%) |
| The reference ranges for the lymphocyte subset analysis are as followings: lymphocyte counts, 400 to 4000 cells/μL; CD3+ T lymphocyte count percentage, 58.4% to 81.6%; CD3+ T lymphocyte count, 770 to 2041 cells/μL; CD4+ T lymphocyte percentage, 30.0% to 46.0%; CD4+ T lymphocyte count, 414 to 1123 cells/μL; CD8+ T lymphocyte percentage, 19.2% to 33.6%; CD8+ T lymphocyte count, 238 to 874 cells/μL; CD4+/CD8+ ratio, 0.68 to 2.47; CD16+ and/or CD56+ positive NK percentage, 5.17% to 24.65%; CD16+ and/or CD56+ positive NK count, 150 to 1000 cells/μL; CD19+ B lymphocyte percentage, 6.48% to 16.6%; CD19+ B lymphocyte count 50 to 670 cells/μL. | |||||||
| 5 | 1245 | 969 (77.83) | 674 (54.13) | 216 (17.35) | 3.12 | 184 (14.78) | 94 (7.55) |
| 39 | 1308 | 1022 (78.13) | 723 (55.26) | 255 (19.50) | 2.84 | 147 (11.24) | 97 (7.42) |
| 68 | 1269 | 1023 (80.61) | 724 (57.05) | 207 (16.31) | 3.5 | 144 (11.35) | 87 (6.86) |
| 83 | 1483 | 1212 (81.72) | 916 (61.77) | 242 (16.32) | 3.79 | 143 (9.64) | 112 (7.55) |
To determine whether the persistent shedding was due to lack of effective antibody response, we conducted serological testing at different time points during her second hospitalization. As shown in Fig. 1C, low levels of IgM presented in the samples of day 40 and day 43, which waned to baseline level by day 73. The IgG antibody level was higher than IgM, waning in the similar pattern. Neutralizing antibody was also measured using a surrogate virus neutralization test (sVNT) based on antibody-mediated blocking of the receptor (ACE)-spike protein (RBD) interaction[3]. The overall neutralizing antibody level was low with an effective titer of 1:10 to 1:20 (Fig. 1D), as determined by the percentage inhibition at 50% to 70% defined by a previous study[3].
From the day 129 on, 8 continuous nucleic acid tests of sputum samples revealed negative results. After a consensus was reached by the expert group in the hospital, the patient was discharged on day 137 after symptom onset. The patient was healthy at the follow-up of two months and the results of viral RNA tests were all negative.
Discussion
Duration of viral shedding and infectivity are paramount for the control and treatment of the infectious disease. As for SARS-CoV-2, temporal pattern analysis of viral shedding showed that the peak of viral shedding appeared at the time of symptom onset[4]. The median duration of viral shedding was 17 days for COVID-19 patients out of Wuhan[5]. However, the viral shedding of SARS-CoV-2 may continue even though symptoms end[6]. Zhou et al reported a COVID-19 case with a duration of up to 37 days[7]. Recently, Liu et al described a case of prolonged viral shedding in which the qRT-PCR for sputum specimens was tested positive for more than 60 days[8]. To our knowledge, so far, our case has the longest duration of SARS-CoV-2 viral shedding: for more than 4 months.
Lin et al investigated the factors associated with the duration of viral shedding among COVID-19 patients and found that the highest temperature at admission, time from symptoms onset to admission and length of hospitalization were risk factors[5]. Interestingly, the patient in our study had no fever at admission and was hospitalized after 4 days from symptom onset. The first hospitalization lasted for 15 days, which was not very long. The patient was healthy and had no underlying diseases. At present, the cause of her condition was still unclear. A plausible explanation was that her IgG level was very low. Timely immune therapy is very important to control COVID-19[9]. The convalescent sera were used to treat 5 critical COVID-19 patients successfully in Shenzhen, China[10]. However, the patient in our study definitely rejected plasma therapy, even though the convalescent sera from the recovered patients had been prepared with high neutralizing antibody titers.
Although the possibility of reinfection could not be excluded, our data strongly suggested it was unlikely. Firstly, there were no new clinical symptoms; secondly, no rise of antibodies (IgM or IgG) was found after the second hospitalization; thirdly, she had no opportunity for re-exposure to SARS-CoV-2 after the first hospitalization as all three family contacts were negative. Actually, the mechanism underlying the long-time viral shedding in COVID-19 cases is unclear. The most important question is whether the patient is shedding live or dead virus. Wölfel et al indicated that the isolation of live virus failed when taking samples after 8 days of illness onset[6]. Liu et al suggested that a small amount of viable virus might exist, even though it was not confirmed by virus culture[8]. In our case, viral culture was performed in BSL-3 laboratory of CDC of Jiangsu Province and live virus was not isolated. Furthermore, all three family members of this patient remained healthy after living together in the same household for 15 to 19 days and their results in antibody screening for SARS-CoV-2 infection were also negative. Therefore, despite being positive for viral RNA in the patient's sputum, it was unlikely that she was shedding infectious viruses.
In conclusion, viral RNA shedding in the sputum of COVID-19 patients may last for over 4 months. As no evidence of infectious virus has been found, two-consecutive negative nucleic acid tests may not be prerequisite for ending quarantine in COVID-19 patients with prolonged viral shedding.
Contributor Information
Yongxiang Yi, Email: yongxiangyinj@163.com.
Lin-Fa Wang, Email: linfa.wang@duke-nus.edu.sg.
References
- 1.National Health Commission, National Administration of Traditional Chinese Medicine Diagnosis and treatment protocol for novel coronavirus pneumonia (Trial Version 7) Chin Med J. 2020;133(9):1087–1095. doi: 10.1097/CM9.0000000000000819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu RJ, Zhao X, Li J, et al Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan CW, Chia WN, Qin X, et al A SARS-CoV-2 surrogate virus neutralization test (sVNT) based on antibody-mediated blockage of ACE2-spike (RBD) protein-protein interaction. Nat Biotechnol. 2020;38(9):1073–1078. doi: 10.1038/s41587-020-0631-z. [DOI] [PubMed] [Google Scholar]
- 4.He X, Lau EHY, Wu P, et al Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26(5):672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 5.Qi L, Yang Y, Jiang DX, et al Factors associated with the duration of viral shedding in adults with COVID-19 outside of Wuhan, China: a retrospective cohort study. Int J Infect Dis. 2020;96:531–537. doi: 10.1016/j.ijid.2020.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wölfel R, Corman VM, Guggemos W, et al Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 7.Zhou F, Yu T, Du RH, et al Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu WD, Chang SY, Wang JT, et al Prolonged virus shedding even after seroconversion in a patient with COVID-19. J Infect. 2020;81(2):318–356. doi: 10.1016/j.jinf.2020.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gasparyan AY, Misra DP, Yessirkepov M, et al Perspectives of immune therapy in coronavirus disease 2019. J Korean Med Sci. 2020;35(18):e176. doi: 10.3346/jkms.2020.35.e176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen CG, Wang ZQ, Zhao F, et al Treatment of 5 Critically Ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323(16):1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]


