Abstract
One of the most widely used programs for detecting positive selection, at the molecular level, is the program codeml, which is implemented in the Phylogenetic Analysis by Maximum Likelihood (PAML) package. However, it has a limitation when it comes to genome-wide studies, as it runs on a gene-by-gene basis. Furthermore, the size of such studies will depend on the number of orthologous genes the genomes have income and these are often restricted to only account for instances where a one-to-one relationship is observed between the genomes. In this work, we present GWideCodeML, a Python package, which runs a genome-wide codeml with the option of parallelization. To maximize the number of analyzed genes, the package allows for a variable number of taxa in the alignments and will automatically prune the topology to fit each of them, before running codeml.
Keywords: Positive selection, Comparative genomics, Genome analysis, Molecular evolution, Protein sequence analysis, Python
With the rise of the genomic era, comparative analyses are gaining interest and are becoming more feasible due to the increase of available genomes. Testing evolutionary hypotheses to measure selective pressure in coding sequences is a common approach in evolutionary biology projects. To do this, there are different bioinformatic tools and resources like the PAML package (Yang 2007). Within this package, codeml allows estimating the ratio (ω) between non-synonymous and synonymous substitutions in protein-coding sequences. The assumption that synonymous mutations accumulate neutrally, implies that ω can be used as a measure of the selective pressure on the coding sequence. In a neutral evolutionary scenario, this ratio will remain equal to one while it will be less or greater than one under purifying and positive selection, respectively. Codeml can be run with different assumptions, including the most used: ω is constant along the whole coding sequence but it can vary among branches (branch model), ω remains constant among branches but it can differ among codon sites in a coding sequence (site model) or assuming that ω varies both among branches and sites (branch-site model). The desired model, along with other parameters, is given to codeml through the control file, along with the coding-sequence alignment of orthologous genes and the species tree.
Implementing codeml in an automatic workflow for a genome-wide approach has its challenges. Among these is the negative correlation between the number of genomes and the number of orthologs shared among them implying that increasing the number of genomes to improve the statistical power of the method will result in reducing the number of analyzed genes. However, the lack of one particular gene, in any of the analyzed species, might not be critical for the analysis if the number of remaining orthologs is substantial.
In this work, we provide a Python package, GwideCodeML, that can be used for running codeml on a set of orthologous coding sequences under site, branch or branch-site models. This package automatically generates the files necessary to run the codeml program including pruning of the topology to match each of the alignments in cases where some taxa are missing compared to the species tree. A dataset of two Saccharomyces species is used for testing our program. The package allowed the use of several outgroup species without decreasing the number of genes included in the analysis.
Methods
Input files
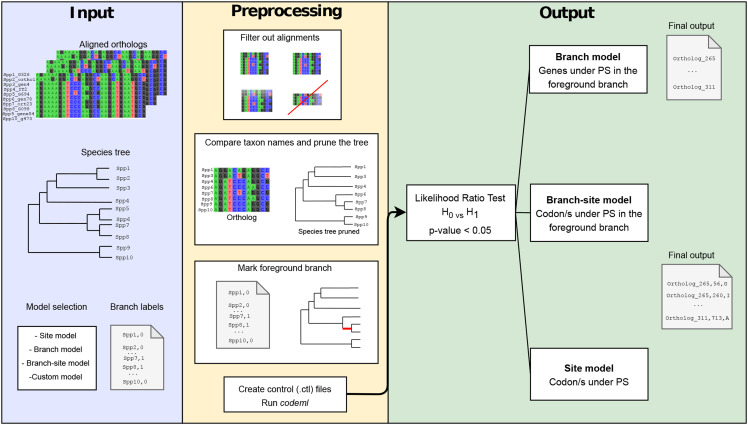
GWideCodeML requires as input, a directory with codon-aligned orthologous sequences in FASTA format and a NEWICK-format tree topology containing all the species included in the analysis (Figure 1). A common denominator between the names within the FASTA file and the taxa (e.g., species and/or strain names) within the tree, is also required. It should be noted that the pipeline itself does not handle possible duplication events and the users should make sure the FASTA files contain a maximum of one sequence per species/strain. Besides, the users need to provide optional configuration parameters such as the model to be tested. Furthermore, it is possible to set a minimum number of species/strains, either belonging to the foreground branch and/or to the outgroup, to filter out alignments with low statistical power. Whether the users decide to test a branch, branch-site model, or to set a threshold, a text file with a list of branch label information is also required as input. In the case of branch and branch-site model, an integer should be added after the branch label to indicate which clade the taxon belongs to. Specifying integers other than 0 and 1 - used for the background and foreground, respectively - will allow for multiple branch testing. In these cases, the workflow will be run for each of the specified foreground clades (integers greater than 0).
Figure 1.
Schematization of the GwideCodeML workflow divided in the three main parts: required input, preprocessing steps necessary for creating control codeml files necessary for testing both null and alternative hypotheses. The last part of the pipeline shows the output file obtained after running LRTs on the codeml results file. This output file contains all the candidate genes of being under positive selection.
Pipeline execution
Codon-aligned sequences are passed through the workflow if: i) only one gene per taxon is found and ii) they pass the threshold of the number of sequences, which by default is set to 0. Hereafter, sequence names are compared between the alignment file and the species tree. The tree will be pruned until it only contains the taxa present within the alignment.
From this step onwards, the workflow performance will depend on the model selected by the user (branch, site, branch-site, or custom). In the case of the branch or branch-site model, the new tree created (pruned or original) will be used to indicate the foreground branch. Hereafter, the control files necessary to run both the alternative and null hypotheses are created and codeml (included in the PAML v4.9 package) is run with each of them. This is the most time-consuming step, however, the package allows parallelization of this task when the user provides a maximum number of threads to be used by the program set by the -p optional parameter. Once codeml has finished, GWideCodeML parses the output files to perform a Likelihood Ratio Test (LRT) to assess the level of significance between the two hypotheses. This last part is optional, allowing the users to analyze codeml results by themselves or letting the pipeline do it for them. When branch or branch-site models have been selected, GWideCodeML will optionally (-gene_trees option) run FastTree (Price et al. 2010) on each of the alignments and compare the topologies of the gene and species tree focusing on the foreground branches. In cases where the studied clade is not monophyletic between the two trees, the user will be informed.
Nested models implemented in the pipeline
Three nested models have been implemented in GWideCodeML for their execution. The branch model allows dN/dS ratio to vary among branches assuming this ratio remains constant among codons (Yang 1998; Yang and Nielsen 1998). This model is useful for detecting positive selection acting on a particular branch and it uses M0 (one-ratio model) and two-ratios model (Goldman and Yang 1994) as null and alternative hypothesis testing, respectively.
Site model assumes dN/dS ratio might be different among codons of the coding sequence provided (Nielsen and Yang 1998; Yang 2000). To test this, model M1a or NearlyNeutral and M2a or PositiveSelection (Yang and Nielsen 1998; Yang et al. 2005) are built-in on the workflow as null and alternative hypotheses, respectively. Finally, the branch-site model is a combination of the two previous approaches as it allows dN/dS ratio to vary among branches and codon sites. Model A null and model A have been used in our package to test both null and alternative hypotheses (Yang et al. 2005; Zhang et al. 2005).
The three described models are nested and therefore a LRT can be applied to assess the level of significance of the null hypothesis. In cases where the p-value obtained from the LRT is lower than 0.05, the null hypothesis is rejected and this gene is further investigated. Additionally, it is possible to vary the level of significance by applying the Bonferroni’s test correction (Miller 1981) when multiple branches are tested. This method has been proved to be effective and usable (Anisimova and Yang 2007) in the correction of the level of significance for null hypothesis rejection depending on the number of hypotheses tested.
Rejecting the null hypothesis means that the alternative hypothesis is more likely than null for the given species topology and alignment. In addition to the LRT, it is necessary to check whether dN/dS ratio is greater than one in the foreground branch (branch or branch-site models) to determine if a gene is a candidate gene of being under positive selection. Moreover, in the cases of site and branch-site models, codeml offers two methods for calculating posterior probabilities for site classes to identify codons under positive selection when the LRT is significant. These methods are naïve empirical Bayes (NEB) and Bayes empirical Bayes (BEB) (Yang et al. 2005). PAML authors highly recommend ignoring NEB output and use BEB instead so the package extracts codon positions with a probability greater than 0.9 of being under positive selection according to BEB results when the LRT is significant.
Additional options and modules
GWideCodeml contains a number of additional scripts and options which can aid the users in preparing the input files or acquire further information about the data. They include i) a module which allows the users to align their codon sequences with three different aligners (Mafft, Muscle or Prank). ii) another module which helps the users to create the alignment necessary to reconstruct a robust species phylogeny by selecting the genes containing sequences for all species included in the analysis. iii) The workflow works with the provided species phylogeny, however, it is possible to set the parameter -gene_tree, and the program will flag the genes where the studied clade is not monophyletic in the species and gene trees. iv) In the case of the branch and branch-site models, the option -dnds will parse the foreground and background ω’s into a separate output file. v) a module which performs a Bonferroni’s test correction when multiple branch testing has been performed.
Output files
The final output of the pipeline is a text file with a list containing genes under positive selection according to the analysis performed. Additionally, when the site or branch-site model is selected, gene names are accompanied with the codon positions in the alignment under positive selection. A gene may have one or more codon positions under positive selection.
Case-study
A genome dataset of two species of Saccharomyces, S. cerevisiae and S. kudriavzevii, was used for testing the performance of our package. These genomes had already been analyzed in a previous work (Macías et al. 2019), where the dataset was conformed of nine genomes: four annotated genomes of each of the Saccharomyces species and Torulaspora delbrueckii as the outgroup species. For testing this package we increased this dataset, particularly the outgroup, with 18 annotated yeast genomes from The Yeast Gene Order Browser (Byrne and Wolfe 2005), a database which also contains information on the homology of different species of the Saccharomycotina subphylum.
In the analysis of selection performed in Macías et al., (2019) with nine genomes, the number of genes analyzed was limited by the number of common orthologous genes shared among them: 4165 genes. In the tested dataset here, which included 26 yeast genomes, only 2753 genes were shared among all species. By setting a minimum number of three background species necessary for testing the evolutionary hypotheses on a gene and four strains in the foreground branch, our pipeline almost doubled the genes analyzed to 4920 genes.
GWideCodeML compared to other software
Several bioinformatics resources have been developed to simplify and/or automate positive selection analyses. Some of these tools are aimed at facilitating the running of codeml and visualize the results in one task, meaning that the users need to run the program gene by gene (Stern et al. 2007; Delport et al. 2010; Steinway et al. 2010; Xu and Yang 2013). There are also other tools created for running codeml in a genome-wide framework like GwideCodeML. Most of these tools have overlapping features among them and when compared to the package presented here (Table 1). The main benefit of our package, when compared to the others, is the combination of features that makes it possible to test site, branch and branch-site models on filtered orthologs with a variable number of sequences among them. PosiGene (Sahm et al. 2017) is the only one of the three published programs which is able to run with a variable number of taxa in the orthologs, it does this by generating a gene tree for each run or as GWideCodeML, prunes the provided species tree to fit each of the alignments. When looking for positive selection, it is important to have a correct topology, however, both species and gene trees have their problems. In the case of gene trees, incorrect topology can occur due to long-branch attraction and natural variation between genes due to the stochastic nature of mutations (Castresana 2007; Jeffroy et al. 2006; Rokas and Carroll 2006). On the other hand, occurrences of horizontal gene transfer will result in the species topology being incorrect. GWideCodeML uses the species tree, but the package will flag the genes where the conservation of the taxa in the studied clade is not the same in the species and gene topology. Furthermore, PosiGene, along with POTION (Hongo et al. 2015), can perform some of the pre-processing steps, although they have been developed to run only one specific model. In contrast, LMAP (Maldonado et al. 2016) is the most flexible regarding the models it offers, the three different nested models along with custom settings, although it lacks the pre-processing steps necessary to run them on a dataset composed of orthologs with a heterogeneous number of sequences. Other features which set GWideCodeML apart from the other three software is that it allows for testing multiple branches in the same run and it contains a module for multiple hypothesis testing.
Table 1. Overlapping features between GWideCodeML and other bioinformatics tools. *1 Built-in models: site model (SM), branch model (BM), branch-site model (BSM). *2 PosiGene generates a new tree for each gene, where GWideCodeML prunes the provided species tree.
| Feature | LMAP | POTION | PosiGene | GWideCodeML |
|---|---|---|---|---|
| Built-in models *1 | SM, BM, BSM | SM | BSM | SM, BM, BSM |
| Run costume models | Yes | — | — | Yes |
| Easy branch labeling | Yes | — | Yes | Yes |
| Automatic pruning *2 | — | — | Yes | Yes |
| Filter out low quality orthologs | — | Yes | Yes | Yes |
| Multithreading | Yes | Yes | Yes | Yes |
Data availability
The GWideCodeML package is implemented in Python and is freely available at https://github.com/lauguma/gwidecodeml
Results And Discussion
This package not only allowed us to analyze a higher number of genes but it has also dramatically increased the statistical power of our study by including more species. If we compared the number of significant genes obtained using only nine genomes and the branch-site model tested here, we can see our package also increased the number of positive results (Table 2). More specifically, we obtained 30 and 32 genes under positive selection in S. kudriavzevii and S. cerevisiae branches, respectively, in Macías et al., (2019). However, using GWideCodeML under the branch-site model assumption, we obtained 137 and 96 in S. kudriavzevii and S. cerevisiae branches, as gene candidates to be under positive selection. Increasing the number of outgroup species has been demonstrated to affect the power of detecting sites under positive selection (Goodswen et al. 2018).
Table 2. Case-study results. Number of detected genes under positive selection after running GwideCodeML twice, one for each branch, using the three built-in nested models. *1. In site models, there is no dN/dS ratio variation among branches, therefore, it was run once.
| Model | Nested models (null vs. alternative hypotheses) | No. genes under positive selection in Sk branch | No. genes under positive selection in Sc branch |
|---|---|---|---|
| Branch | M0 vs. two-ratios | 83 | 31 |
| Branch-site | MAnull vs. MA | 137 | 96 |
| Site | M1a vs. M2a | 32*1 | 32*1 |
Besides, our package facilitates the running of additional models such as branch and site models. The combination of the results of the three approaches tested can provide more depth on how positive selection has been acting on the studied clade.
Acknowledgments
The computations were performed on Tirant III of the Spanish Supercomputing Network (Servei d’Informàtica of the University of Valencia).
Footnotes
Communicating editor: P. Morrell
Literature Cited
- Anisimova M., and Yang Z., 2007. Multiple hypothesis testing to detect lineages under positive selection that affects only a few sites. Mol. Biol. Evol. 24: 1219–1228. 10.1093/molbev/msm042 [DOI] [PubMed] [Google Scholar]
- Byrne K. P., and Wolfe K. H., 2005. The Yeast Gene Order Browser: combining curated homology and syntenic context reveals gene fate in polyploid species. Genome Res. 15: 1456–1461. 10.1101/gr.3672305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castresana J., 2007. Topological variation in single-gene phylogenetic trees. Genome Biol. 8: 216 10.1186/gb-2007-8-6-216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delport W., Poon A. F. Y., Frost S. D. W., and Kosakovsky Pond S. L., 2010. Datamonkey 2010: A suite of phylogenetic analysis tools for evolutionary biology. Bioinformatics 26: 2455–2457. 10.1093/bioinformatics/btq429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman N., and Yang Z., 1994. A codon-based model of nucleotide substitution for protein-coding DNA sequences. Mol. Biol. Evol. 11: 725–736. 10.1093/oxfordjournals.molbev.a040153 [DOI] [PubMed] [Google Scholar]
- Goodswen S. J., Kennedy P. J., and Ellis J. T., 2018. A gene-based positive selection detection approach to identify vaccine candidates using Toxoplasma gondii as a test case protozoan pathogen. Front. Genet. 9: 332 10.3389/fgene.2018.00332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongo J. A., de Castro G. M., Cintra L. C., Zerlotini A., and Lobo F. P., 2015. POTION: An end-to-end pipeline for positive Darwinian selection detection in genome-scale data through phylogenetic comparison of protein-coding genes. BMC Genomics 16: 567 10.1186/s12864-015-1765-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffroy O., Brinkmann H., Delsuc F., and Philippe H., 2006. Phylogenomics: the beginning of incongruence? Trends Genet. 22: 225–231. 10.1016/j.tig.2006.02.003 [DOI] [PubMed] [Google Scholar]
- Macías L. G., Morard M., Toft C., and Barrio E., 2019. Comparative genomics between Saccharomyces kudriavzevii and S. cerevisiae applied to identify mechanisms involved in adaptation. Front. Genet. 10: 187 10.3389/fgene.2019.00187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado E., Almeida D., Escalona T., Khan I., Vasconcelos V. et al. , 2016. LMAP: Lightweight Multigene Analyses in PAML. BMC Bioinformatics 17: 354 10.1186/s12859-016-1204-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. G. J., 1981, pp. 67–70 in Simultaneous statistical inference, Springer-Verlag, New York: 10.1007/978-1-4613-8122-8 [DOI] [Google Scholar]
- Nielsen R., and Yang Z., 1998. Likelihood models for detecting positively selected amino acid sites and applications to the HIV-1 envelope gene. Genetics 148: 929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price M. N., Dehal P. S., and Arkin A. P., 2010. FastTree 2–Approximately Maximum-Likelihood Trees for Large Alignments. PLoS One 5: e9490 10.1371/journal.pone.0009490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokas A., and Carroll S. B., 2006. Bushes in the tree of life. PLoS Biol. 4: e352 10.1371/journal.pbio.0040352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahm A., Bens M., Platzer M., and Szafranski K., 2017. PosiGene: Automated and easy-to-use pipeline for genome-wide detection of positively selected genes. Nucleic Acids Res. 45: e100 10.1093/nar/gkx179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinway S. N., Dannenfelser R., Laucius C. D., Hayes J. E., and Nayak S., 2010. JCoDA: A tool for detecting evolutionary selection. BMC Bioinformatics 11: 284 10.1186/1471-2105-11-284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern A., Doron-Faigenboim A., Erez E., Martz E., Bacharach E. et al. , 2007. Selecton 2007: Advanced models for detecting positive and purifying selection using a Bayesian inference approach. Nucleic Acids Res. 35: W506–W511. 10.1093/nar/gkm382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B., and Yang Z., 2013. PamlX: A graphical user interface for PAML. Mol. Biol. Evol. 30: 2723–2724. 10.1093/molbev/mst179 [DOI] [PubMed] [Google Scholar]
- Yang Z., 1998. Likelihood ratio tests for detecting positive selection and application to primate lysozyme evolution. Mol. Biol. Evol. 15: 568–573. 10.1093/oxfordjournals.molbev.a025957 [DOI] [PubMed] [Google Scholar]
- Yang Z., 2000. Maximum likelihood estimation on large phylogenies and analysis of adaptive evolution in human influenza virus A. J. Mol. Evol. 51: 423–432. 10.1007/s002390010105 [DOI] [PubMed] [Google Scholar]
- Yang Z., 2007. PAML 4: Phylogenetic Analysis by Maximum Likelihood. Mol. Biol. Evol. 24: 1586–1591. 10.1093/molbev/msm088 [DOI] [PubMed] [Google Scholar]
- Yang Z., and Nielsen R., 1998. Synonymous and nonsynonymous rate variation in nuclear genes of mammals. J. Mol. Evol. 46: 409–418. 10.1007/PL00006320 [DOI] [PubMed] [Google Scholar]
- Yang Z., Wong W. S. W., and Nielsen R., 2005. Bayes Empirical Bayes Inference of Amino Acid Sites Under Positive Selection. Mol. Biol. Evol. 22: 1107–1118. 10.1093/molbev/msi097 [DOI] [PubMed] [Google Scholar]
- Zhang J., Nielsen R., and Yang Z., 2005. Evaluation of an improved branch-site likelihood method for detecting positive selection at the molecular level. Mol. Biol. Evol. 22: 2472–2479. 10.1093/molbev/msi237 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The GWideCodeML package is implemented in Python and is freely available at https://github.com/lauguma/gwidecodeml