Abstract
Some semelparous organisms in nature mate as many times as they can in a single reproductive episode before death, while most iteroparous species including humans avoid such suicidal reproductive behavior. Animals naturally pursue more sex and the possible fatal consequence of excessive sex must be orchestrated by negative feedback signals in iteroparous species, yet very little is known about the regulatory mechanisms. Here we used Drosophila male sexual behavior as a model system to study how excessive sex may kill males and how the nervous system reacts to prevent death by sex. We found that continuous sexual activity by activating the fruitless-expressing neurons induced a fixed multi-step behavioral pattern ending with male death. We further found negative feedback in the fly brain to prevent suicidal sexual behavior by expression changes of the neurotransmitters acetylcholine and gamma-aminobutyric acid, and neuropeptide F. These findings are crucial to understand the molecular underpinnings of how different organisms choose reproductive strategies and balance reproduction and survival.
Electronic supplementary material
The online version of this article (10.1007/s12264-020-00604-5) contains supplementary material, which is available to authorized users.
Keywords: Drosophila, Reproduction, Survival, NPF, GABA, Acetylcholine
Introduction
Most species, including humans, balance sexual behaviors and survival, avoiding excessive sexual activity based on their past experience and internal physiological state [1–3]; however, there are some semelparous organisms, such as the insectivorous marsupials, that mate as many times as they can in a single reproductive episode that eventually kills them [4]. While the evolutionary mechanism of suicidal reproduction in semelparous animals is of much interest, it is hard to study the molecular and neuronal mechanisms underlying such behavior due to the very limited genetic and neurological tools in these animals.
Drosophila melanogaster is iteroparous with survival and reproduction well balanced [2]. Male courtship in Drosophila is a well-studied innate behavior, which is largely controlled by the sex-specific transcription factors (FRUM and DSXM in males, and DSXF in females) encoded by the fruitless (fru) and doublesex (dsx) genes [5–7]. FRUM is responsible for most aspects of male courtship [8–10], and is expressed in a dispersed subset of ~2000 neurons including sensory neurons, interneurons, and motor neurons that are interconnected to form sex circuitry [9–12]. Recently, much progress has been made on how sensory cues are integrated by the male-specific P1 neurons that initiate male courtship, and how experiences may alter P1 excitability to modulate courtship [13–20]. In particular, mating experience decreases further courtship through dopamine modulation of P1 neurons [2, 21, 22], providing a potential mechanism to avoid excessive sexual behavior in male flies. However, there is no evidence that sexual activity per se in male flies perturbs their survival, although exposure to female pheromones does [23, 24], making it hard to study the molecular and neuronal mechanisms underlying suicidal reproduction despite the advanced genetic tools in this species [25].
We previously showed that mild activation of all fruM neurons induces courtship behaviors in solitary males, which perform courtship behaviors until death [26]. In this study, we used two genetic models, one in the above males with fruM neurons activated that are semelparous, and the other in wild-type males that are iteroparous. Combining these two genetic models, we investigated how excessive sexual activity accelerates death in male flies, and how the central nervous system reacts to prevent such behaviors. We found that continuous performance of sexual behaviors induces a fixed behavioral pattern ending with male death. We further found a negative feedback in the fly brain involving acetylcholine, gamma-aminobutyric acid (GABA) and neuropeptide F (NPF) to prevent suicidal sexual behavior.
Materials and Methods
Fly Stocks
Flies were maintained at 22°C or 25°C in a 12h:12h light:dark cycle. Canton-S flies were used as the wild-type strain. fruGAL4 [10], fruLexA [27], UAS-dTrpA1 (II) [28], UAS-CsChrimson (attp2) [29], UAS-fruMi (attp2) [30], UAS-fruMiScr (attp2) [30], R19B03-Gal4 (attp2) [31], NPF-GAL4 (BDSC#25682), and Crz-GAL4 (BDSC#51976) have been described previously. RNAi lines (UAS-GABA-B-R3-RNAi, UAS-Dsk-RNAi, UAS-CCKLR-17D3-RNAi, UAS-Octbeta-2R-RNAi, UAS-NPF-RNAi, UAS-NPFR1-RNAi, UAS-Trh-RNAi, and UAS-Dop1R1-RNAi all at attp2, and UAS-Gad1-RNAi and UAS-ChAT-RNAi at attp40) have been described previously [32] and were from Tsinghua Fly Center at Tsinghua University.
Male Courtship Assay
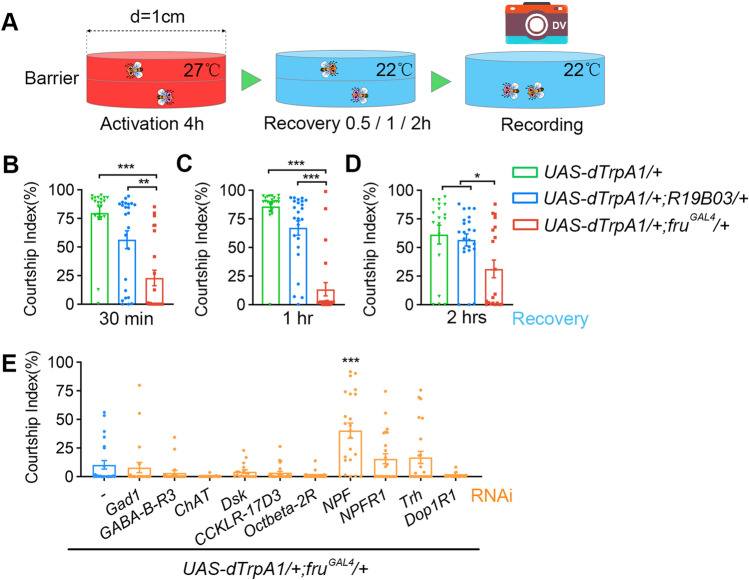
In courtship assays for Fig. 4, 4–8-day-old wild-type virgin females were loaded individually into cylindrical chambers (diameter: 1 cm; height: 3 mm per layer) as courtship targets, and 4–6-day-old test males were then gently loaded into the chambers after cold anesthesia and separated from target females by a transparent film. The chambers were warmed at 27°C for 4 h allowing dTRPA1-mediated neuronal activation, and transferred to 22°C for 30 min, 1 h, or 2 h before the 10-min courtship test. The courtship index (CI), which is the percentage of observation time a male fly performs courtship, was used to measure courtship towards female targets, and measured manually using LifeSongX software.
Fig. 4.
NPF represses courtship after excessive sexual output. A Schematic for experiments assaying courtship inhibition after 4 h of continuous sexual output. B–D Reduced male courtship toward virgin females after 4 h of continuous activation of fruM neurons. For 0.5 h recovery, n = 21, 24, and 23, P < 0.001, Kruskal-Wallis test. **P < 0.01, ***P < 0.001, post hoc Dunn’s multiple comparisons test. For 1 h recovery, n = 22, 24, and 23, P < 0.001, Kruskal-Wallis test. ***P < 0.001, post hoc Dunn’s multiple comparisons test. For 2 h recovery, n = 18, 23, and 20, P = 0.032, Kruskal-Wallis test. *P < 0.05, post hoc Dunn’s multiple comparisons test. E Knocking down NPF partially restores male courtship that was suppressed by 4-h activation of fruM neurons. n = 24, 22, 20, 24, 18, 23, 22, 23, 24, 24, and 22. ***P < 0.001, Mann Whitney U test compared with control without RNAi (blue). Error bars indicate SEM.
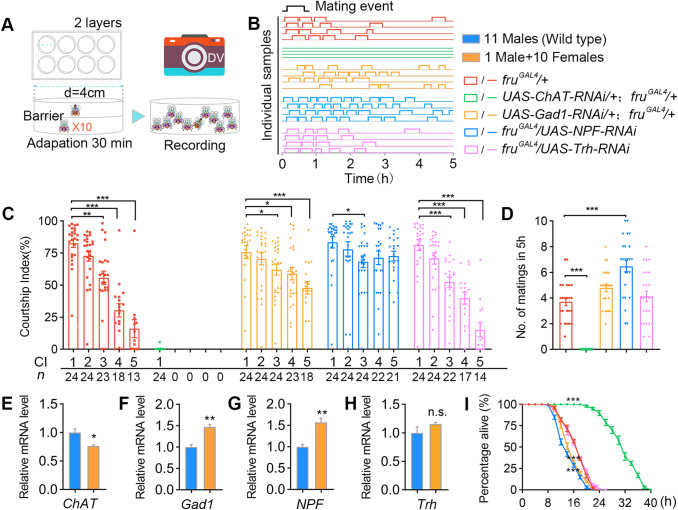
In the courtship assays for Fig. 5, 4–8-day-old wild-type virgin females were loaded into large cylindrical chambers (diameter: 4 cm; height: 3 mm per layer) as courtship targets (10 females per chamber), and 4–6 day-old-test males were then individually loaded into chambers after cold anesthesia, and separated from target females by a transparent film until courtship testing for 5 h continuously at 25°C.
Fig. 5.
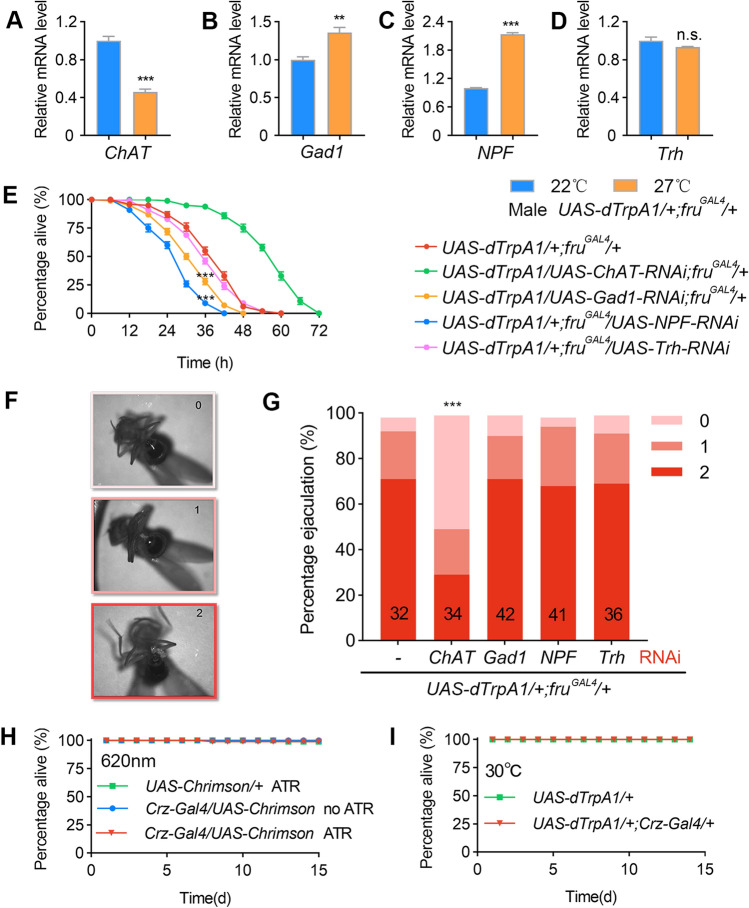
GABA and NPF inhibit excessive sexual activity in wild-type males. A Behavioral setup allowing up to 10 matings (1M+10F) in a 5-h test. B Examples of male mating activity in 5 h. Genotypes as indicated by colors below. C Courtship indices of males in the 1M+10F environment. CI-1 indicates courtship in the first 10 min after the male and females are introduced; CI-2, CI-3, CI-4, and CI-5 indicate 10-min courtship after the 1st, 2nd, 3rd, and 4th matings, respectively. Kruskal-Wallis test, *P < 0.05, **P < 0.01, ***P < 0.001, post hoc Dunn’s multiple comparisons test. N as indicated below. D Numbers of matings by males during the 5-h test. Knockdown of NPF significantly increases the number of matings. n = 24 per group, P < 0.001, one-way ANOVA, ***P < 0.001, post hoc Tukey’s multiple comparisons test. E–H Wild-type males housed with 10 virgin females have reduced chat expression and elevated Gad1 and NPF expression in their brains compared with groups of 11 males. Flies were housed in vials without food for 12 h at 25°C. N = 3 per group. n.s., not significant, *P < 0.05, **P < 0.01, unpaired t-test. I Knocking down Gad1 or NPF accelerates, while knocking down ChAT slows down death in males housed with 10 virgin females without food. n = 10 for each. Each n consists of 10 vials of 1M+10F. ***P < 0.001 at 16 h, Mann Whitney U test. Error bars indicate SEM.
Feeding Assay
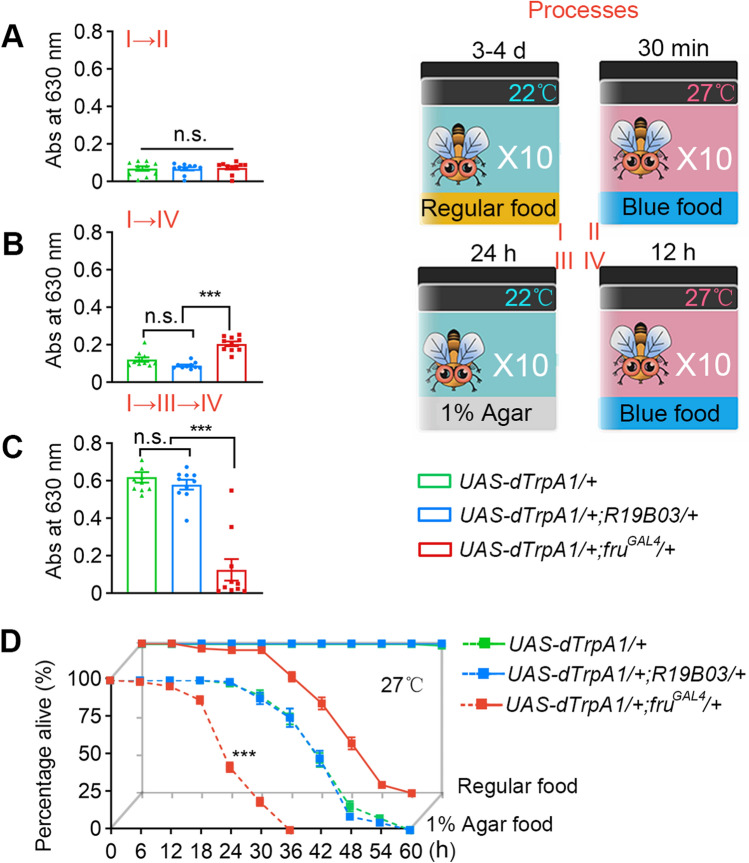
Feeding was assayed using food with a blue dye. In brief, in a regular feeding assay test (Fig. 2C), flies were starved for 24 h on 1% aqueous agar food at 22°C, then transferred to 1% FD & C Blue 1 food (2.5% sucrose, 2.5% yeast extract, and 0.5% agar; Sigma-Aldrich, St. Louis, MO) at 27°C for 30 min (the food was pre-warmed at 27°C for 3 h). The feeding test shown in Fig. 2A was the same but used non-starved males; that in Fig. 2B used non-starved males and allowed feeding at 27°C for 12 h. To quantify food intake, the absorbance of the ingested blue dye was measured at 630 nm using a 96-well microplate spectrophotometer.
Fig. 2.
Abnormal feeding in continuously courting males. A–C Males with activated fruM neurons eat more in the short term, but much less in the long term. Feeding assays are illustrated on the right. n = 10 (10 flies/group) for each. n.s., not significant, ***P < 0.001, Kruskal–Wallis test, post hoc Dunn’s multiple comparisons test. D Feeding alleviates but does not prevent death in males with activated fruM neurons. n = 10 (10 flies/group) for each. ***P < 0.001 at 24 h, Mann Whitney U test. Error bars indicate SEM.
Survival Assay
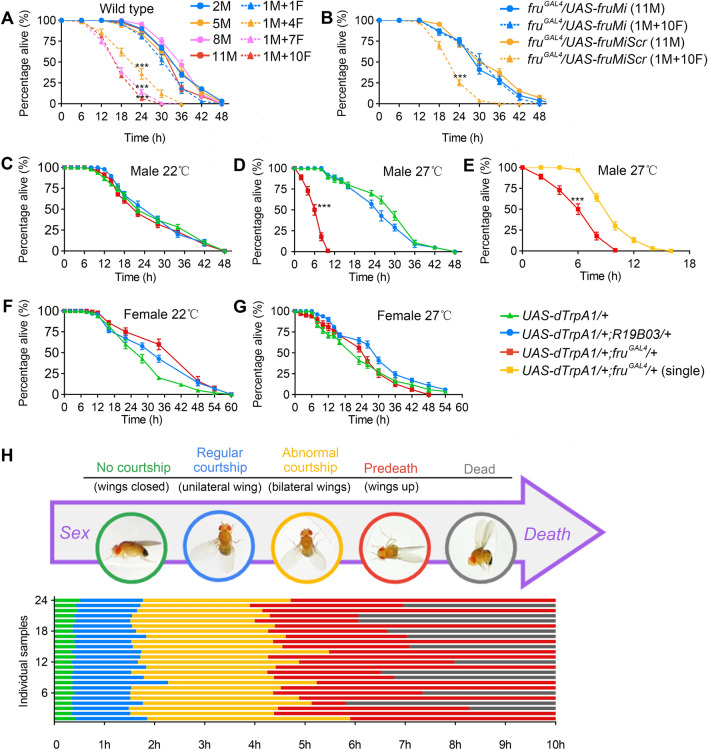
Adult male flies were collected within 12 h after eclosion and group housed (10–15 males) for 3-4 days before the survival test. In the survival assay for Figs 1A, B, and 5I, individual test males were loaded with a certain number of virgin females (1M+1F, 1M+4F, 1M+7F, or 1M+10F) into empty vials without food, and controls with the same group size of males (2M, 5M, 8M, or 11M). The survival of males was scored every 6 h at 25°C. Only the 1M+10F condition was used for the survival assays shown the Figs 1B and 5I. The sample size for all experiments was 10, and each sample contained 8–11 males. For all M+F groups (1M+1F, 1M+4F, 1M+7F or 1M+10F), 10 vials were used as one sample, thus there were 100 males in each group. For the 2M group, 5 vials were used as one sample (100 males; for the 5M group, 2 vials were used as one sample (100 males; and for the 8M and 11M groups, each vial was a sample (80 and 110 males).
Fig. 1.
Coupling sex and death in Drosophila males. A Survival rates of wild-type males under conditions allowing different levels of sexual activity. Males die much sooner when group-housed with increasing numbers of virgin females (M, males; F, virgin females). Flies were housed in vials without food and assayed at 25°C. N = 10 for each. Each n consists of 10 vials of 1M+1F, 1M+4F, 1M+7F, 1M+10F, 5 vials of 2M, 2 vials of 5M, or one vial of 8M or 11M. ***P < 0.001 for 1M+4F vs 5M, 1M+7F vs 8M, or 1M+10F vs 11M at the 24-h time point, Mann Whitney U test. B Males with fruM knocked down survive similarly when grouped with 10 virgin females or in groups of 11 males. n = 10 for each. Each n consists of 10 vials of 1M+10F or one vial of 11M. ***P < 0.001, Mann Whitney U test. C, D Mildly activating all frum neurons at 27°C induces continuous courtship and accelerates male death. All males were housed in groups of 10 without food. n = 10 for each. ***P < 0.001 at 6 h, Mann Whitney U test. E Males with fruM neurons activated die much more quickly in groups of 10 males than in isolation. n = 10 for each. Each n consists of one vial of 10 males or 10 vials of single-housed males. ***P < 0.001 at 6 h, Mann Whitney U test. F, G Activating fruM counterpart neurons in females does not accelerate female death. n = 10 (10 females/group) for each. H Activating fruM neurons induces a series of stereotypical behavior patterns from regular courtship display to male death. Flies were individually housed in round chambers without food and recorded at 27°C. n = 24. Error bars indicate SEM.
The survival assay shown in the Fig. S1 was almost the same as above, but 1% agar + 1% sugar medium in vials at 22°C was provided. The number of dead males was scored twice a day. Flies were transferred to new vials with medium every 2 days.
In the survival assays for Figs 1C–G, 2D, and 3E, I: groups of 10 males (or 10 females in Fig. 1F, G) were housed in vials without food (Fig. 1C–G), or in a nutritional environment (1% agar for the 3 agar groups in Fig. 2D; regular food for the other 3 groups in Figs 2D and 3E, I) at specified temperatures. Flies housed in vials with food were transferred to new food vials every 2 days, and dead flies were removed and counted. Ten replicate vials were established for each group, a total of 100 flies.
Fig. 3.
Counterbalancing molecular changes induced by excessive sexual output. A–D Continuous activation of fruM neurons decreases chat expression and increases Gad1 and NPF expression in male brains. Groups of 10 males were housed in food vials for 12 h at 27°C. n = 3 per group. n.s., not significant, **P < 0.01, ***P < 0.001, unpaired t-test. E Knocking down chat alleviates, while knocking down Gad1 or NPF further accelerates, the fatal effect of fruM neuronal activation in males. All test males were housed in food vials in groups of 10 at 27°C. n = 10 (10 flies/group) for each. ***P < 0.001 at 36 h, Mann Whitney U test. F Evaluation of ejaculation in individual males. 0 refers to no ejaculation, 1 and 2 refer to ejaculation with different volumes. G Knocking down chat significantly reduces the level of ejaculation in males with activated fruM neurons. n as indicated inside bars. ***P < 0.001, χ2 test. H, I Activation of Crz neurons via light-sensitive CsChrimson (H) or temperature-sensitive dTrpA1 (I) does not affect mortality over a 15-day period on regular food or food with ATR. n = 10 each, each n consists of 10 flies. Error bars indicate SEM.
In the survival assays for Fig. 3H, flies were raised on regular food or food with 0.2 mmol/L ATR (all-trans-retinal, MFCD00001550, Sigma-Aldrich, St. Louis, MO) throughout development and adulthood in the dark. Test males were group-housed (10 flies per vial) and exposed to 620-nm red light (40 Hz, 8 ms duration, 0.071 mW/mm2). Flies were transferred to new vials containing fresh food every 2 days, and dead flies were removed and counted. Ten replicate vials were established for each group (total, 100 males).
Ejaculation Assay
To evaluate ejaculation, males were loaded individually into cylindrical chambers (diameter, 2 cm; height, 3 mm) after ice anesthesia, and allowed to recover at 22°C for 30 min, then transferred to 27°C for 30 min. Ejaculation levels (0, 1, or 2; see Fig. 3F) were manually scored under a microscope.
Body Weight
A few 1.5-mL centrifugal tubes were numbered and each weighed as M0. Then 20 males were loaded into each tube after CO2 anesthesia, and weighed as M1. The same 20 males were transferred from the centrifugal tube to an empty vial, transferred to 27°C for 6 h, and then returned to the original centrifugal tube and weighed as M2. The percentage weight loss was (M2-M1)/(M1-M0)×100%. Five replicates were measured for each group.
Quantitative Real-time PCR
Fly samples from neuronal activation and mating experiments were frozen in liquid nitrogen and decapitated by vigorous vortexing. The heads were then separated from the bodies using metal sieves. Each sample, consisting of 30 frozen heads, was used for total RNA using TRIzol reagent (15596026, Thermo Fisher Scientific, Waltham, MA). We purified total RNA using a DNA-freeTM Kit (AM1906, Thermo Fisher Scientific, Waltham, MA) and performed reverse transcription using SuperScriptTM IV (18091050, Thermo Fisher Scientific, Waltham, MA) to obtain cDNA used for templates. Quantitative PCR was performed on the Roche LightCycler® 96 Real-Time PCR machine using AceQ qPCR SYBR Green Master Mix (Q121-02, Vazyme, Nanjing). Transcript levels were analyzed by the 2^-ΔCT method using Actin as an internal control. Each sample was run in triplicate. Each experiment was repeated three times using independent sets of genetic crosses. Primers used for RT-PCR quantification were:
- Actin Forward
5′-GTCGCGATTTAACCGACTACCTGA-3′
- Actin Reverse
5′-TCTTGCTT CGAGATCCACATCTGC-3′
- ChAT Forward
5′-TGAATATGCCTTGAGCTGTGC-3′
- ChAT Reverse
5′-TCGTCGAGAATTCCGCAAAC-3′
- Gad1 Forward
5′-GTGCCACCACATTGAAGTACC-3′
- Gad1 Reverse
5′-AGACCGTTGGACAGCTGATTG-3′
- Trh Forward
5′-TCCATTCTACACACCAGAACCG-3′
- Trh Reverse
5′-ACTGGGCAAAACTGGAGTTG-3′
- NPF Forward
5′-CCTCATTAAAACGCGAGCAAAT-3′
- NPF Reverse
5′-ATCGCTGATGGATATCCTGAGG-3′
Tissue Dissection, Staining, and Imaging
Brains of 4–6 day-old males and females were dissected in Schneider’s insect medium (S2) and fixed in 4% paraformaldehyde in 0.5% Triton X-100 and 0.5% bovine serum albumin in phosphate-buffered saline (PAT) for 30 min at room temperature. After 4 × 10-min washes, tissues were blocked in 3% normal goat serum (NGS) for 60 min, then incubated in primary antibodies diluted in 3% NGS for 4 h at room temperature and 1–2 days at 4°C, then washed in PAT, and incubated in secondary antibodies diluted in 3% NGS for 4 h at room temperature and 1–2 days at 4°C. The tissues were then washed thoroughly in PAT and mounted for imaging. The antibodies used were rabbit anti-NPF (1:500; RB-19-0001, RayBiotech, Norcross, GA), rabbit anti-GFP (1:1000; A11122, Invitrogen, Waltham, MA), and secondary Alexa Fluor 488 antibodies (1:500, A21206, Invitrogen, Waltham, MA). Samples were imaged at ×20 magnification on Zeiss 710 confocal microscopes using ZEN (https://www.zeiss.com/microscopy/int/products/microscope-software/zen.html) and processed with Fiji software (https://imagej.net/Fiji/Downloads).
Statistics
Experimental flies and genetic controls were tested under the same conditions, and data are collected from at least two independent experiments. Statistical analysis was performed using GraphPad Prism 8 (https://www.graphpad.com/scientific-software/prism/) as indicated in each figure legend. Data were first verified for normal distribution by the D’Agostino–Pearson normality test. For normally-distributed data, Student’s t test was used for pairwise comparisons, and one-way ANOVA was used for comparisons among multiple groups, followed by Tukey’s multiple comparisons. For data not normally-distributed, the Mann-Whitney U test was used for pairwise comparisons, and the Kruskal–Wallis test was used for comparison among multiple groups, followed by Dunn’s multiple comparisons. For RT-PCR experiments, the average relative expression of three independent experiments was analyzed using the unpaired t-test. The χ2 test was used to compare two groups in the male ejaculation assay.
Results
Coupling Sex and Death: A Drosophila Model
Previous studies revealed that male courtship behavior per se does not affect life span in male Drosophila [23, 24]. We wondered if the excessive expression of courtship behaviors (successive mating in a short period of time) would be costly, and tested the survival rate of individual wild-type Canton-S (wtcs) males housed with increasing numbers of virgin females (1M+1F, 1M+4F, 1M+7F, or 1M+10F). We also tested the survival rates of groups of males with the same group size as above (2M, 5M, 8M, or 11M). We found that male flies grouped with 4, 7, or 10 virgin females died more quickly than those grouped with males or with only one female, if no food was provided (Fig. 1A). To confirm that this effect was induced by sexual activity, we used male flies with fruM knockdown by expressing RNAi targeting fruM [30] using fruGAL4, which rarely showed any courtship behavior when grouped with females during the 2-day test period. We found that such males had the same survival rates when grouped with 10 virgin females or in groups of 11 males (Fig. 1B). We also tested groups of wtcs flies (1M+10F, or 11M) under low nutrition conditions (1% agar + 1% sugar), and found that individual males housed with 10 females died more quickly than groups of 11 males (Fig. S1). However, we did not find a survival difference in males housed with females (1M+10F) or males (11M) within a 30-day period if regular food was provided. These results indicate that excessive sexual activity with multiple females is costly to male survival under low nutrition conditions but has no effect on survival if fed on regular food.
Although the above results revealed an effect of excessive sexual activity on survival, wild-type male flies would not court until death. We previously showed that activation of ~2,000 fruM-expressing neurons at 29°C using the warmth-activated cation channel dTRPA1 [28] induces continuous courtship behaviors and eventually kills males [26]. Thus we used such a model to couple sex and death in male flies, and study how excessive sexual activity would be fatal and how flies avoid such behavior. We tested the survival rate of groups of 11 males without food, and found that all showed similar survival rates at the permissive temperature (22°C, Fig. 1C); however, half of the UAS-dTrpA1/+; fruGAL4/+ males at 27°C performing continuous courtship behaviors died in ~6 h, much more quickly than control UAS-dTrpA1/+ males (Fig. 1D). We used 27°C, rather than the 29°C previously used [26], as it allowed much longer survival of UAS-dTrpA1/+; fruGAL4/+ males, suitable for further genetic manipulations (see below). We also tested the survival rate of male flies in which ~2,000 mushroom body (MB) neurons (labeled by R19B03) were activated at 27°C, and did not find any survival deficit (Fig. 1D). Furthermore, individual UAS-dTrpA1/+; fruGAL4/+ males survived longer than groups of such males, probably due to the lack of energy-consuming chasing behaviors that were only found in groups of such males (Fig. 1E). We found no survival deficit in UAS-dTrpA1/+; fruGAL4/+ females at 27°C with all fruGAL4 neurons activated (Fig. 1F, G). These results indicated that it is the continuous output of sexual behaviors, but not the continuous activation of a large number of neurons (MB neurons in males and females; fruGAL4 neurons in females), that kills flies in hours.
To reveal how continuous sexual activity results in quick death in males, we recorded individual UAS-dTrpA1/+; fruGAL4/+ males at 27°C for 10 h, and found a stereotypic behavioral expression from sex to death in all males: initially they closed their wings and did not show any courtship in the first few minutes after transfer to 27°C, and performed intensive courtship behaviors including unilateral wing extension and abdominal bending for 1–2 h. Later, all males began to extend and vibrate the wings bilaterally, indicating abnormal courtship, for 2–3 h, followed by a pre-death phase when males stopped walking, raised both wings, and bent their abdomen to attempt copulation, until motionless and dead (Figs 1H and S2).
As sexual activity is energy-consuming, we tested how 6-h continuous expression of courtship behaviors might change body weight. We found that after 6-h activation of fruM neurons at 27°C, males lost ~20% of their body weight, while 6-h activation of fruM neurons in females, or 6-h activation of MB neurons in males or females, like control UAS-dTrpA1/+ males or females, lost <10% of their body weight (Fig. S3A–D), which may be due to dehydration during this period. That 6-h activation of fruM neurons induces ~10% more weight loss in males than control flies indicates that continuous sexual activity is associated with energy expenditure that burns ~10% of their body weight in 6 h.
Feeding Alleviates but Does not Prevents Death From Sex
The above results show how continuous activation of fruM neurons accelerates male death if food is not provided, and we next tested whether these males could eat, and if so, if feeding could prevent death. We first transferred fed males to 27°C, allowing feeding on blue food for 30 min. Males with activated fruM neurons or MB neurons, or control UAS-dTrpA1/+ males, all showed similarly low levels of feeding (Fig. 2A). However, when we transferred fed males to 27°C and allowed 12-h feeding on blue food, we found that males with activated fruM neurons ate more than males with activated MB neurons or control UAS-dTrpA1/+ males (Fig. 2B), which may be due to the higher energy consumption and need for food in males with activated fruM neurons. In contrast, when starved males were allowed 12-h feeding on blue food at 27°C, those with activated fruM neurons ate significantly less than males with activated MB neurons or control UAS-dTrpA1/+ males (Fig. 2C). These results demonstrate that while performing intensive courtship behaviors, males with activated fruM neurons can eat, so feeding and courtship behaviors are not exclusive at least in this context, although the level of feeding is much lower than control males over 12 h. Indeed, males with activated fruM neurons at 27°C survived much longer if provided food [Fig. 2D, 50% of males survived for >36 h with regular food, and ~18 h with 1% agar, compared to ~6 h without any food (Fig. 1D)]. Thus males with activated fruM neurons have a lower level of feeding, and such feeding alleviates but does not prevent death.
Altered Neural Transmission Upon Excessive Sexual Activity
As flies, like most animals, do not court and mate until death, we reasoned that there must be feedback signals upon excessive sexual activity to inhibit further such behaviors. We first used real-time PCR to assess changes in the mRNA expression of major neurotransmitters, as well as neuropeptide F (NPF) that is a reward signal for mating [33, 34], in the brains of UAS-dTrpA1/+; fruGAL4/+ males after 12-h at 27°C. We found reduced expression of choline acetyltransferase (ChAT), increased expression of the GABA synthesis enzyme glutamic acid decarboxylase 1 (Gad1), and NPF, and unaltered expression of tryptophan hydroxylase (Trh) (Fig. 3A–D). These results indicated that continuous sexual activity resulted in reduced excitatory acetylcholine (ACh) signaling, and enhanced inhibitory GABA signaling, as well as elevated NPF signaling. To test if these expression changes play a role in survival during the activation of fruM neurons, we knocked down ChAT, Gad1, NPF, or Trh in UAS-dTrpA1/+; fruGAL4/+ males using RNAi [32], and assayed their survival rates at 27°C on food. We found that the lethal effect of activating fruM neurons was alleviated by knockdown of ChAT, but accelerated by knockdown of Gad1 or NPF (Fig. 3E). Together, these results indicate that feedback neuromodulation (reduced ACh and increased GABA and NPF) by continuous sexual activity is protective and enhances male survival.
We noted that all the above males at 27°C performed intensive courtship behaviors in groups of 10 males, and found no difference, so we further tested the ejaculation rates of individual males at 27°C. We found that knockdown of ChAT, but not Gad1, NPF, or Trh, significantly decreased the ejaculation rates of UAS-dTrpA1/+; fruGAL4/+ males (Fig. 3F, G), suggesting a role of ACh-dependent ejaculation in the mortality of these males. To test if excessive ejaculation alone kills males, we activated Corazonin (Crz) neurons, which induce ejaculation but no other courtship behaviors in isolated males [34, 35], and found no survival deficit during the 15-day test period using either optogenetic (Fig. 3H) or thermogenetic activators (Fig. 3I). Together, these results suggest that continuous display of courtship behaviors other than ejaculation (wing extension and abdominal bending) is more fatal to males, although ejaculation might enhance this fatal effect.
Negative Feedback Prevents Continuous Sexual Activity
To test if prior experience of excessive sexual activity inhibits further courtship behaviors, we first kept individually-housed UAS-dTrpA1/+; fruGAL4/+ males at 27°C for 4 h, allowing continuous courtship expression, then transferred them to 22°C for 30 min, 1 h, or 2 h, during which all males stopped courting. We then introduced virgin females to test male courtship behavior after the above experience (Fig. 4A). We found that males with 4-h experience of fruM neuronal activation, even after 0.5–2 h recovery, showed much reduced courtship of virgin females (Fig. 4B–D; the percentage of time males displayed any courtship behavior was ~13% after 1 h of recovery, compared to ~85% in control males). We reasoned that the altered neural transmission described above might be involved in courtship inhibition after continuous courtship expression, so we separately knocked down ChAT, Gad1, NPF, Trh, and some other transmission related genes in UAS-dTrpA1/+; fruGAL4/+ males. All males tested showed reduced courtship levels (compared to the control CI of ~85%) after 4-h continuous courtship expression and 1-h recovery; however, we found that knocking down NPF in fruM-expressing neurons significantly alleviated the courtship inhibition effect (Fig. 4E; the CI was ~40% with NPF knockdown, and ~10% with intact NPF). Together, these results indicate that excessive sexual activity induces expression changes of certain transmission genes including NPF, which in turn inhibit further courtship behaviors.
The above results used males with fruM neurons forcefully activated for 4 h, but whether this applied to regular males without artificial neuronal activation was uncertain. Thus we placed individual males with 10 virgin females, and recorded their courtship behaviors for 5 h (Fig. 5A). We found that control fruGAL4/+ males, as well as males with Gad1, NPF, or Trh knocked down, mated multiple times; however, males with ChAT knocked down in fruM neurons rarely courted and never mated (Fig. 5B). Further analysis of their courtship behaviors showed that control fruGAL4/+ males, as well as those with Trh knocked down, reduced their further courtship after each mating, such that their CI was ~15% after 4 successive matings (Fig. 5C, CI-5). Although males with Gad1 knocked down reduced their courtship after mating, this reduction was not as severe as in control males (Fig. 5C, CI-5; ~48% after 4 successive matings). Surprisingly, males with NPF knocked down displayed indistinguishable levels of courtship even after 4 successive matings (Fig. 5C; initial CI-1 ~83%, and CI-5 after 4 matings ~73%, not significantly different). Furthermore, while control fruGAL4/+ males and those with Trh knocked down had an average of ~4 successive matings with 10 virgin females within 5 h (3.7 ± 0.3 and 4.1 ± 0.4, respectively), males with NPF knocked down showed more successive matings (6.5 ± 0.5, Fig. 5D). Males with Gad1 knocked down showed slightly more successive matings (4.8 ± 0.3), but not significantly different from controls (Fig. 5D). Together, these results reveal crucial roles of GABA and NPF in inhibiting further sex after excessive sexual experiences.
As we showed that knockdown of NPF in fruM neurons eliminated the courtship inhibition effect after excessive sexual activity, we tried to identify the specific NPF and fruM co-expressing neurons involved in courtship inhibition. Anti-NPF staining in male and female brains revealed three pairs of male-specific NPF neurons (Fig. S4A, B). These neurons were also faithfully labeled through genetic intersectional labelling between fruLexA and NPF-GAL4 (Fig. S4C, D). Consistent with our findings, a recent study uncovered the role of these male-specific NPF neurons in inhibiting courtship [36].
We further found that, after 5 h of experience with 10 virgin females, the mRNA level of ChAT decreased, while those of Gad1 and NPF increased in the brains of wild-type males (Fig. 5E-H), which is consistent with our previous findings in males with activated fruM neurons. We then measured the survival rate of males housed in the 1M+10F environment, and found that males with ChAT knocked down that rarely courted survived much longer than control fruGAL4/+ males, while males with Gad1 or NPF knocked down died slightly quicker (Fig. 5I), probably due to their continuously high level of courtship and mating. Thus, using two independent genetic models, we showed that ACh, GABA, and NPF are key modulators that respond to excessive sexual activity, and prevent continuous sex that may result in male death.
Discussion
In this study, we established a model in which Drosophila males perform continuous sexual behaviors until death, mimicking the behavior of semelparous animals during the mating season. We also identified ACh, GABA, and NPF as key feedback modulators in the brain that respond to excessive sexual activity and prevent semelparous mating.
Previous studies imply that mating does not affect lifespan in Drosophila [23, 24]. We showed that while single mating (1M+1F) does not affect survival, multiple matings (1M+4F, 1M+7F, and 1M+10F) do affect male survival, at least under lower nutrition conditions, and this is probably due to the energy expenditure incurred during courtship display and mating. In an extreme example, males with activated fruM neurons performed intense courtship behaviors for 6 h and lost ~10% of their body weight, compared to males that did not perform courtship behaviors. However, whether this effect is specific to sexual behaviors or could also be caused by other kinds of strenuous physical activity still needs further investigation.
A striking phenomenon was that while males with activated fruM neurons at 27°C performed intensive courtship behaviors, they were able to eat, although significantly less, consistent with a previous finding that sexually-aroused males with ~23 pairs of male-specific P1 neurons activated have reduced feeding [37]. This phenomenon indicates that sexual and feeding behaviors are not mutually exclusive. Indeed, males with activated fruM neurons at 27°C survived ~30 h longer if provided food (median survival time ~36 h with food, and 6 h without food).
Males of most species decrease further sexual activity after mating to prevent excessive sex; such a process must evolve feedback signals that respond to mating, or changes in metabolism or immunity, and decrease sexual drive through the nervous system. Indeed, we found a spontaneous negative feedback mechanism used by male flies to protect them from excessive sex. On one hand, the enzyme for the generally excitatory neurotransmitter ACh, ChAT, was lower and the enzyme for the inhibitory neurotransmitter GABA, Gad1, was higher in males with activated fruM neurons or experienced with 10 virgin females. On the other hand, knockdown of ChAT in fruM neurons alleviated, and knockdown of Gad1 in fruM neurons accelerated death in the above males. These results reveal how the nervous system responds to excessive sexual activity by re-balancing the excitatory and inhibitory signals.
We also found that the neuropeptide NPF as an important modulator that prevents excessive sexual activity. First, NPF expression was increased in males with activated fruM neurons or males housed with 10 virgin females, consistent with previous notions that NPF is a reward signal for mating [33, 34]. Second, knockdown of NPF partially restored courtship behaviors in males with fruM neurons activated for 4 h. Furthermore, wild-type males with NPF knocked down failed to decrease courtship even after 4 successive matings, such that they could mate up to 10 times (the maximum, as there were 10 females in total) in 5 h. Third, knockdown of NPF accelerated the deaths caused by fruM neuronal activation or grouping with 10 females. Thus, while NPF functions as a reward signal for mating, its cumulative increase after mating experiences inhibits further sex. Recently, Liu and colleagues found that disrupting NPF signaling increases sexual activity, and identified subsets of NPF and fruM co-expressing male-specific neurons that inhibit courtship [36]. Meanwhile, another recent study by Zhang and colleagues found NPF signaling to be courtship-promoting, although they were involved in recurrent circuitry for sexual satiety [22]. Our results are generally consistent with the former study that NPF functions as a repressor of courtship, at least when it becomes excessive, although these studies used different behavioral protocols. Future study of how NPF possesses dual roles, first as a reward signal, then an inhibitor for further sex, probably through distinct NPF and/or NPFR neurons, would help to interpret these discrepancies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the Tsinghua Fly Center and Bloomington Stock Center for the fly stocks. This work was supported by grants from the National Key R&D Program of China (2019YFA0802400), the National Natural Science Foundation of China (31970943, 31622028, and 31700920), and the Jiangsu Innovation and Entrepreneurship Team Program.
Conflict of interest
All authors claim that there are no conflicts of interest.
Footnotes
Can Gao and Chao Guo are contributed equally to this work.
References
- 1.Rodriguez-Manzo G. Glutamatergic transmission is involved in the long lasting sexual inhibition of sexually exhausted male rats. Pharmacol Biochem Behav. 2015;131:64–70. doi: 10.1016/j.pbb.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Zhang SX, Rogulja D, Crickmore MA. Dopaminergic circuitry underlying mating drive. Neuron. 2016;91:168–181. doi: 10.1016/j.neuron.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 3.Phoenix CH, Chambers KC. Old age and sexual exhaustion in male rhesus macaques. Physiol Behav. 1988;44:157–163. doi: 10.1016/0031-9384(88)90132-1. [DOI] [PubMed] [Google Scholar]
- 4.Fisher DO, Dickman CR, Jones ME, Blomberg SP. Sperm competition drives the evolution of suicidal reproduction in mammals. Proc Natl Acad Sci U S A. 2013;110:17910. doi: 10.1073/pnas.1310691110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burtis KC, Baker BS. Drosophila doublesex gene controls somatic sexual differentiation by producing alternatively spliced mRNAs encoding related sex-specific polypeptides. Cell. 1989;56:997–1010. doi: 10.1016/0092-8674(89)90633-8. [DOI] [PubMed] [Google Scholar]
- 6.Ryner LC, Goodwin SF, Castrillon DH, Anand A, Villella A, Baker BS, et al. Control of male sexual behavior and sexual orientation in Drosophila by the fruitless gene. Cell. 1996;87:1079–1089. doi: 10.1016/S0092-8674(00)81802-4. [DOI] [PubMed] [Google Scholar]
- 7.Ito H, Fujitani K, Usui K, Shimizu-Nishikawa K, Tanaka S, Yamamoto D. Sexual orientation in Drosophila is altered by the satori mutation in the sex-determination gene fruitless that encodes a zinc finger protein with a BTB domain. Proc Natl Acad Sci U S A. 1996;93:9687–9692. doi: 10.1073/pnas.93.18.9687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demir E, Dickson BJ. Fruitless splicing specifies male courtship behavior in Drosophila. Cell. 2005;121:785–794. doi: 10.1016/j.cell.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 9.Manoli DS, Foss M, Villella A, Taylor BJ, Hall JC, Baker BS. Male-specific fruitless specifies the neural substrates of Drosophila courtship behaviour. Nature. 2005;436:395–400. doi: 10.1038/nature03859. [DOI] [PubMed] [Google Scholar]
- 10.Stockinger P, Kvitsiani D, Rotkopf S, Tirian L, Dickson BJ. Neural circuitry that governs Drosophila male courtship behavior. Cell. 2005;121:795–807. doi: 10.1016/j.cell.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 11.Cachero S, Ostrovsky AD, Yu JY, Dickson BJ, Jefferis GS. Sexual dimorphism in the fly brain. Curr Biol. 2010;20:1589–1601. doi: 10.1016/j.cub.2010.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu JY, Kanai MI, Demir E, Jefferis GS, Dickson BJ. Cellular organization of the neural circuit that drives Drosophila courtship behavior. Curr Biol. 2010;20:1602–1614. doi: 10.1016/j.cub.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 13.Inagaki HK, Jung Y, Hoopfer ED, Wong AM, Mishra N, Lin JY, et al. Optogenetic control of Drosophila using a red-shifted channelrhodopsin reveals experience-dependent influences on courtship. Nat Methods. 2014;11:325–332. doi: 10.1038/nmeth.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clowney EJ, Iguchi S, Bussell JJ, Scheer E, Ruta V. Multimodal chemosensory circuits controlling male courtship in Drosophila. Neuron. 2015;87:1036–1049. doi: 10.1016/j.neuron.2015.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kallman BR, Kim H, Scott K. Excitation and inhibition onto central courtship neurons biases Drosophila mate choice. Elife. 2015;4:e11188. doi: 10.7554/eLife.11188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen D, Sitaraman D, Chen N, Jin X, Han C, Chen J, et al. Genetic and neuronal mechanisms governing the sex-specific interaction between sleep and sexual behaviors in Drosophila. Nat Commun. 2017;8:154. doi: 10.1038/s41467-017-00087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan Y, Meissner GW, Baker BS. Joint control of Drosophila male courtship behavior by motion cues and activation of male-specific P1 neurons. Proc Natl Acad Sci U S A. 2012;109:10065–10070. doi: 10.1073/pnas.1207107109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu S, Guo C, Zhao H, Sun M, Chen J, Han C, et al. Drosulfakinin signaling in fruitless circuitry antagonizes P1 neurons to regulate sexual arousal in Drosophila. Nat Commun. 2019;10:4770. doi: 10.1038/s41467-019-12758-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimura K, Hachiya T, Koganezawa M, Tazawa T, Yamamoto D. Fruitless and doublesex coordinate to generate male-specific neurons that can initiate courtship. Neuron. 2008;59:759–769. doi: 10.1016/j.neuron.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Ho MS. A shared neural node for multiple innate behaviors in Drosophila. Neurosci Bull. 2018;34:1103–1104. doi: 10.1007/s12264-018-0311-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang SX, Miner LE, Boutros CL, Rogulja D, Crickmore MA. Motivation, perception, and chance converge to make a binary decision. Neuron. 2018;99(376–388):e376. doi: 10.1016/j.neuron.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang SX, Rogulja D, Crickmore MA. Recurrent circuitry sustains Drosophila courtship drive while priming itself for satiety. Curr Biol. 2019;29(3216–3228):e3219. doi: 10.1016/j.cub.2019.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harvanek ZM, Lyu Y, Gendron CM, Johnson JC, Kondo S, Promislow DEL, et al. Perceptive costs of reproduction drive ageing and physiology in male Drosophila. Nat Ecol Evol. 2017;1:152. doi: 10.1038/s41559-017-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flintham EO, Yoshida T, Smith S, Pavlou HJ, Goodwin SF, Carazo P, et al. Interactions between the sexual identity of the nervous system and the social environment mediate lifespan in Drosophila melanogaster. Proc Biol Sci 2018, 285: 20181450. [DOI] [PMC free article] [PubMed]
- 25.Guo C, Pan Y, Gong Z. Recent advances in the genetic dissection of neural circuits in Drosophila. Neurosci Bull. 2019;35:1058–1072. doi: 10.1007/s12264-019-00390-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan Y, Robinett CC, Baker BS. Turning males on: activation of male courtship behavior in Drosophila melanogaster. PLoS One. 2011;6:e21144. doi: 10.1371/journal.pone.0021144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan Y, Baker BS. Genetic identification and separation of innate and experience-dependent courtship behaviors in Drosophila. Cell. 2014;156:236–248. doi: 10.1016/j.cell.2013.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, Jegla TJ, et al. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klapoetke NC, Murata Y, Kim SS, Pulver SR, Birdsey-Benson A, Cho YK, et al. Independent optical excitation of distinct neural populations. Nat Methods. 2014;11:338–346. doi: 10.1038/nmeth.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meissner GW, Luo SD, Dias BG, Texada MJ, Baker BS. Sex-specific regulation of Lgr3 in Drosophila neurons. Proc Natl Acad Sci U S A. 2016;113:E1256–1265. doi: 10.1073/pnas.1600241113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jenett A, Rubin GM, Ngo TT, Shepherd D, Murphy C, Dionne H, et al. A GAL4-driver line resource for Drosophila neurobiology. Cell Rep. 2012;2:991–1001. doi: 10.1016/j.celrep.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ni JQ, Liu LP, Binari R, Hardy R, Shim HS, Cavallaro A, et al. A Drosophila resource of transgenic RNAi lines for neurogenetics. Genetics. 2009;182:1089–1100. doi: 10.1534/genetics.109.103630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shohat-Ophir G, Kaun KR, Azanchi R, Mohammed H, Heberlein U. Sexual deprivation increases ethanol intake in Drosophila. Science. 2012;335:1351–1355. doi: 10.1126/science.1215932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zer-Krispil S, Zak H, Shao L, Ben-Shaanan S, Tordjman L, Bentzur A, et al. Ejaculation induced by the activation of Crz neurons is rewarding to Drosophila Males. Curr Biol. 2018;28(1445–1452):e1443. doi: 10.1016/j.cub.2018.03.039. [DOI] [PubMed] [Google Scholar]
- 35.Tayler TD, Pacheco DA, Hergarden AC, Murthy M, Anderson DJ. A neuropeptide circuit that coordinates sperm transfer and copulation duration in Drosophila. Proc Natl Acad Sci U S A. 2012;109:20697–20702. doi: 10.1073/pnas.1218246109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu WW, Ganguly A, Huang J, Wang YJ, Ni JFD, Gurav AS, et al. Neuropeptide F regulates courtship in Drosophila through a male-specific neuronal circuit. Elife. 2019;8:29. doi: 10.7554/eLife.49574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang W, Guo C, Chen D, Peng Q, Pan Y. Hierarchical control of Drosophila sleep, courtship, and feeding behaviors by male-specific P1 neurons. Neurosci Bull. 2018;34:1105–1110. doi: 10.1007/s12264-018-0281-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.