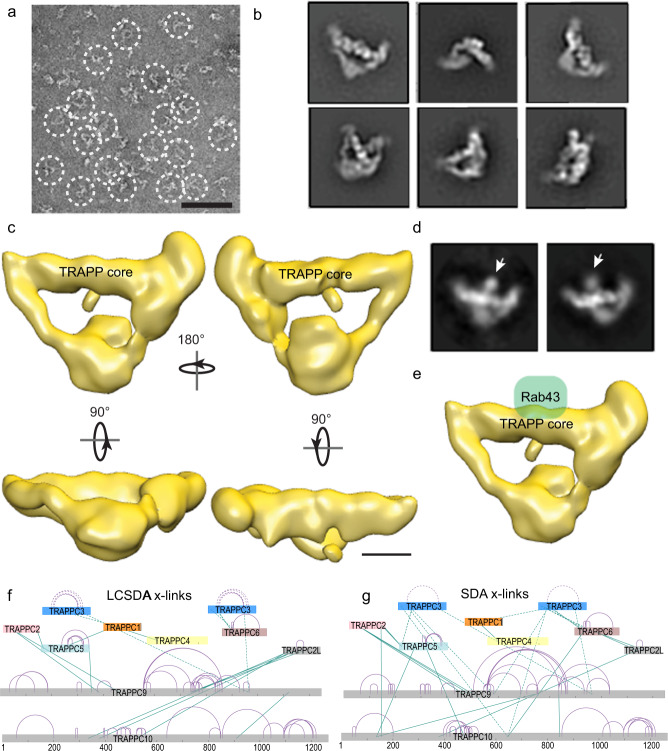
Fig. 4. Electron microscopy and chemical cross-linking reveal architecture of the TRAPPII complex.
a A representative raw image of negatively stained purified human TRAPPII complex. Triangle-shaped particles but not diamond-shaped particles were observed (circled). Scale bar is 50 nm. b Representative 2D class averages of TRAPPII complex. Box edge length of 45 nm. c 3D reconstruction of TRAPPII with multiple orientations of the complex. Scale bar is 50 Å. d Representative 2D class averages of TRAPPII complex with GST-tagged Rab43. The Rab43 density localizes to the center of the TRAPP core (arrow). Box edge length of 45 nm. e EM density maps of TRAPPII with a modeled position of Rab43. Scale bar is 50 Å. f, g Reduced set of cross-linking mass spectrometry data for the TRAPPII complex using the hetero-bifunctional photo-activatable cross-linkers NHS-Diazirine (SDA), and NHS-LC-Diazirine (LC-SDA). The TRAPP subunits are arranged similar as in the orientation of Fig. 1a. Inter-protein cross-links are shown in green, with intra-protein cross-links shown in purple. Cross-links to the TRAPPC3 subunit are shown as dashed lines to highlight the ambiguity arising from two copies. Complete cross-linking data are shown in the source data. Cross-link map networks are generated with xiNET online cross-link viewer tool80.