Abstract
Objective:
Little is known about the optimal treatment of avoidant/restrictive food intake disorder (ARFID). The purpose of this study was to evaluate feasibility, acceptability, and proof-of-concept for cognitive-behavioral therapy for ARFID (CBT-AR) in children and adolescents.
Methods:
Males and females (ages 10–17 years) were offered 20–30 sessions of CBT-AR delivered in a family-based or individual format.
Results:
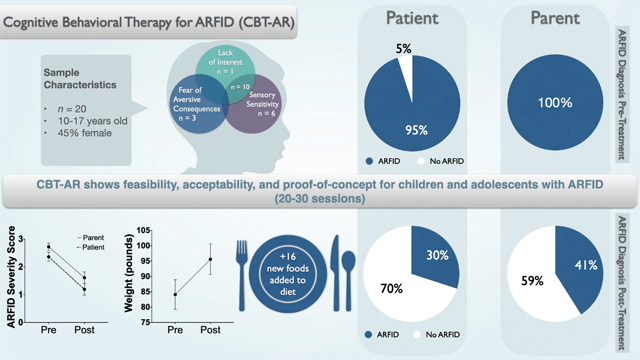
Of 25 eligible individuals, 20 initiated treatment, including 17 completers and three dropouts. Using intent-to-treat analyses, clinicians rated 17 patients (85%) as “much improved” or “very much improved.” ARFID severity scores (on the Pica, ARFID, and Rumination Disorder Interview) significantly decreased per both patient and parent report. Patients incorporated a mean of 16.7 (SD = 12.1) new foods from pre- to post-treatment. The underweight subgroup showed a significant weight gain of 11.5 (SD = 6.0) pounds, moving from the 10th to the 20th percentile for body mass index. At post-treatment, 70% of patients no longer met criteria for ARFID.
Conclusions:
This is the first study of an outpatient manualized psychosocial treatment for ARFID in older adolescents. Findings provide evidence of feasibility, acceptability, and proof-of-concept for CBT-AR. Randomized controlled trials are needed.
Keywords: Avoidant/restrictive food intake disorder, ARFID, cognitive-behavioral therapy, CBT, modular treatment, children, adolescents, sensory sensitivity
Graphical Abstract
Introduction
Avoidant/restrictive food intake disorder (ARFID) was added to the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5) as a reformulation of the DSM-IV diagnosis feeding disorder of infancy and early childhood (APA, 2013). ARFID is a serious mental health condition characterized by avoidance or restriction of food intake that is not motivated by shape or weight concerns. Individuals with ARFID restrict their food intake—by volume and/or variety—due to sensory sensitivity, fear of aversive consequences, and/or lack of interest in eating or food. This can lead to weight loss or faltering growth, nutritional deficiencies, dependence on tube feeding or nutritional supplements, and/or serious disruptions in functioning.
Although ARFID can affect individuals across the lifespan, prevalence and morbidity data focus primarily on youth. One study of 1,444 Swiss schoolchildren aged 8–13 years found that 3.2% reported diagnostic features of ARFID on a self-report questionnaire (Kurz et al., 2015). Data from the United States suggest that individuals with ARFID are increasingly presenting to eating-disorder treatment programs, where they comprise up to 22.5% of patients (Nicely et al., 2014). Individuals with ARFID are at risk for physical health problems such as bradycardia (Norris et al., 2014), menstrual irregularities (Aulinas et al., 2020), and psychiatric comorbidities (Kambanis et al., 2020). In one study, 34% of patients with ARFID required inpatient hospitalization due to low weight and/or unstable vital signs (Norris et al., 2014).
Despite data suggesting that ARFID is prevalent and impairing, there is little evidence to guide treatment. Only three small randomized controlled trials have been published, and all have focused on young children ages 13 months to 12 years (Sharp et al., 2016; Sharp et al., 2017; Lock et al., 2019a). Published research on the treatment of older children and adolescents is limited to retrospective chart reviews (e.g., Bryson et al., 2018; Makhzoumi et al., 2019), case studies (e.g., Thomas et al., 2017), and case series (e.g., Dumont et al., 2019; Rienecke et al., 2020). A growing number of reports have highlighted the potential application of cognitive-behavioral (Gormez et al., 2018; Steen et al., 2018; Zucker et al., 2019), family-based (Eckhardt et al., 2019; Lock et al., 2019b; Rienecke et al., 2020; Spettigue et al., 2018), and parent management approaches (Dahlsgaard et al., 2019). For example, a case series of 11 children and adolescents ages 10–18 years found that CBT delivered in an intensive day-hospital format resulted in significant reductions in ARFID symptoms (Dumont et al., 2019). Similarly, a randomized controlled trial (n = 28) of children ages 5–12 years highlighted the feasibility of outpatient family-based treatment (FBT) in comparison to usual care (Lock et al., 2019a). Although some of these emerging therapies for older children and adolescents have been briefly outlined in book chapters (Fitzpatrick et al., 2015) or journal articles (Dumont et al., 2019; Lock et al., 2019b), none have been comprehensively described in treatment manuals that could facilitate empirical testing or dissemination.
There are evidence-based outpatient protocols for psychiatric disorders that resemble ARFID—including pediatric feeding disorders, eating disorders, and anxiety disorders—but none is appropriate for ARFID specifically. Treatments for pediatric feeding disorders typically focus on infants, toddlers, and preschool children and include interventions that are not developmentally appropriate for older children or adolescents (e.g., Williams & Foxx, 2007). CBT for eating disorders focuses on reducing shape and weight concerns (Fairburn et al., 2008; Waller et al., 2019), which are not central to ARFID (Becker et al., 2019). Treatments for specific phobia target fear of choking (McNally, 1994) or vomiting (Hunter & Antony 2009), but lack interventions to promote weight gain or resolve nutrition deficiencies. In summary, an outpatient treatment that directly targets the core psychopathology of ARFID is urgently needed.
To meet this need, the first cognitive-behavioral treatment manual specifically for individuals with ARFID was recently published. The purpose of the current study was to evaluate feasibility, acceptability, and proof-of-concept for CBT for ARFID (CBT-AR; Thomas & Eddy, 2019) in an open trial of patients ages 10–17 years. The Obesity-Related Behavioral Interventions Trials (ORBIT) model lays out an iterative model of intervention development (Czajkowski et al., 2015). ORBIT defines a proof-of-concept study as an uncontrolled within-subjects study that evaluates whether a novel intervention could plausibly offer clinically significant benefit that could later be evaluated in a randomized controlled trial. We designed our study to test the following key hypotheses. First, regarding feasibility, we hypothesized that the majority of patients offered CBT-AR would take up the treatment. Regarding acceptability, we predicted that patients and their parents would give CBT-AR high ratings of credibility and satisfaction. Regarding proof-of-concept, we hypothesized that therapists would observe significant symptom improvements in their patients, and that patients and parents would report significant reductions in ARFID symptom severity. We further hypothesized that patients would incorporate several new foods and that underweight patients would gain significant weight. We also predicted that many patients would no longer meet criteria for ARFID at post-treatment, and that patients would exhibit significant reductions in anxiety and depression. Lastly, we expected there would be few adverse events.
Methods
Participants
We recruited patients for this treatment trial from the greater Boston area over a two-year period from October 2016 to December 2018 from those who were already participating in an observational study of the neurobiology of ARFID. The neurobiology study included males and females ages 10–23 years with full or subthreshold ARFID as determined by the Pica, ARFID, and Rumination Disorder Interview (PARDI) (Bryant-Waugh et al., 2019). Exclusion criteria were clinically significant disordered eating as evidenced by an Eating Disorder Examination-Questionnaire (Fairburn & Beglin, 2008) global score > 4.0, and/or any self-induced vomiting, laxative use, diuretic use, fasting, or compensatory exercise in the past four weeks. Because the neurobiology study required neuroimaging and endocrine assays, additional exclusion criteria were (1) the use of hormones, pregnancy, or breastfeeding; (2) current psychosis, alcohol use disorder, substance use disorder, or active suicidal or homicidal ideation on the Kiddie Schedule for Affective Disorders and Schizophrenia for School Age Children-Present and Lifetime Version (KSADS-PL; Kaufman et al., 2013); (3) lifetime history of gastrointestinal tract surgery; (4) intellectual disability evaluated by medical history; (5) contraindications for magnetic resonance imaging (MRI) (e.g., braces, history of brain trauma); and (6) hematocrit < 30%. In total, 65 participants took part in the neurobiology study during the treatment trial recruitment period.
In addition to taking part in the neurobiology study, inclusion criteria for the treatment trial included (1) age 10–17 years (as we are recruiting 18–65 year-olds separately for an adult trial); (2) meeting full criteria for ARFID on either the patient or parent version of the PARDI; (3) completing the baseline neurobiology visit; (4) living within one hour’s drive of the clinic; (5) not receiving concurrent psychosocial treatment; (6) no changes in psychotropic medication (if taking) for the past 12 weeks; (7) availability to initiate treatment within four weeks of the baseline visit; and (8) no current tube feeding. Of the 65 who participated in the neurobiology study during the recruitment period, 25 were eligible and offered treatment. Figure 1 depicts participant flow.
Figure 1.
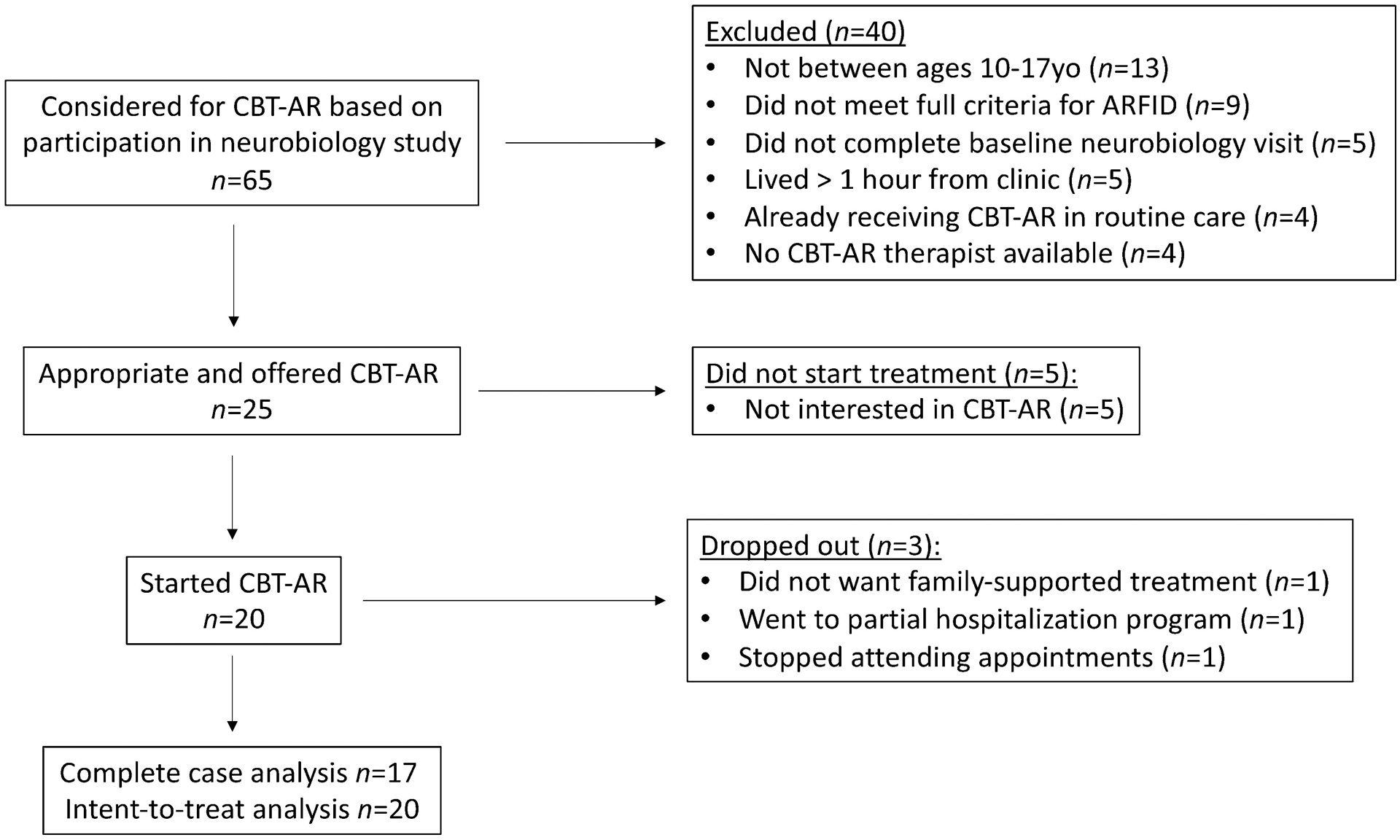
Patient flow diagram for proof-of-concept trial of cognitive-behavioral therapy for avoidant/restrictive food intake disorder (CBT-AR) in children and adolescents
Procedure
The study was registered at www.clinicaltrials.gov (https://clinicaltrials.gov/ct2/show/NCT02963220) and approved by the Partners Human Research Committee prior to recruitment. Because patients were under 18 years old, they provided assent and one or both of their parents provided informed consent.
Patients received the study treatment at no cost at the Massachusetts General Hospital Eating Disorders Clinical and Research Program. Depending on the components completed, they were paid $100–250 for the pre-treatment evaluation including a three-hour screening visit and an eight-hour baseline neurobiology visit (blood draws, MRI, stool samples, behavioral tasks, interviews, and self-report questionnaires). They were then paid up to $200–300 for completing weekly questionnaires and another eight-hour neurobiology visit after completing CBT-AR. Because the current paper focuses on feasibility, acceptability, and proof-of-concept, we do not report the neurobiology findings here. Research assistants who were not therapists in the trial conducted all assessments.
Treatment
Manualized CBT-AR is a flexible, modular treatment comprising 20–30 sessions over four stages: (1) psychoeducation and early change; (2) treatment planning; (3) addressing maintaining mechanisms; and (4) preventing relapse. In Stage 1, the therapist provides psychoeducation about ARFID, encourages the patient to establish regular eating and self-monitoring, and helps the patient increase food volume (if underweight) and/or variety (if not underweight) (Thomas & Eddy, 2019). In Stage 2, the therapist provides psychoeducation about nutrition deficiencies and supports the patient in selecting novel foods to learn about in Stage 3 that will support resolution of these deficiencies, encourage further weight gain (if underweight), and/or reduce clinical impairment. In Stage 3, the therapist selects one, two, or three module(s) appropriate to the patient’s ARFID maintaining mechanisms(s). Although Stage 3 interventions differ based on module, common elements include in-session exposure and between-session practice. Patients with sensory sensitivity taste five novel foods per session; patients with fear of aversive consequences create and work through a fear and avoidance hierarchy; and patients with lack of interest in eating or food practice interoceptive exposures. Lastly, in Stage 4, the therapist evaluates progress and co-creates a relapse prevention plan.
CBT-AR is available in one of two formats based on patient age and weight status. Specifically, we provided family-supported CBT-AR (in which one or both parents attended all sessions) to all patients younger than 16 years old, and to patients ages 16–17 who were also underweight. We provided individual CBT-AR to patients age 16 and older who were normal-weight or overweight. Although session attendees differed by format, interventions remained the same across the developmental spectrum.
We offered patients up to 20 sessions (if not underweight) and up to 30 sessions (if underweight, to provide additional support for weight gain). However, because this was an initial proof-of-concept study, we offered patients the flexibility to terminate the treatment early if goals had been achieved, or to extend treatment if treatment goals had not yet been met. Sessions lasted 50 minutes and were provided weekly in Stages 1, 2, and 3, and every 2–3 weeks in Stage 4. The treatment was provided by one of three PhD therapists (JJT, KRB, or KTE). Two of the three therapists were the authors of the treatment manual and the third was trained and supervised by the manual authors. Study therapists met weekly for peer supervision. Therapists audio-recorded all sessions so that an independent PhD rater (REL) could make fidelity (ranging from 1—“not at all adherent” to 7—“completely adherent”) and competence (ranging from 1—“not at all” to 5—“completely”) ratings based on published measures (Thomas & Eddy, 2019). Ratings of two randomly selected sessions for each of the first ten patients—including sessions from all three therapists—indicated high levels of fidelity (M = 6.3, SD = 0.8) and competence (M = 5.0, SD = 0.0).
Patients received concurrent medical monitoring from a pediatrician or adolescent medicine physician. For patients who were underweight (BMI < 10th percentile) or had exhibited significant weight loss that would qualify them as meeting criterion A1 for ARFID, the physician set an individualized target weight range that would return the patient to his or her pre-illness BMI growth trajectory. A subset (n = 8) were also followed by a psychiatrist or other healthcare professional because they had been taking a stable dose of psychotropic medication. (This subset includes one patient who started a selective serotonin reuptake inhibitor shortly after his baseline visit, but was retained in the study because he had already initiated CBT-AR.) Patients were not allowed to receive any other interventions—such as nutrition counseling, occupational therapy, speech therapy, or other forms of psychotherapy—during the trial.
Measures
Pica, ARFID, and Rumination Disorder Interview (PARDI).
The PARDI is a structured interview of the specific psychopathology of ARFID (Bryant-Waugh et al., 2019). The PARDI can be used to confer ARFID diagnoses and also contains an overall severity scale assessing constructs central to the ARFID diagnosis including dietary variety across the five food groups (dairy, grains, proteins, fruits, vegetables), nutrition deficiencies, underweight, reluctance to try novel foods, and psychosocial impairment. The PARDI also provides profile severity scores for each of three ARFID profiles corresponding to the three rationales for food restriction and avoidance described in DSM-5, including sensory sensitivity, fear of aversive consequences, and lack of interest in eating or food. PARDI scale scores range from 0 (no symptoms) to 6 (extreme severity). The PARDI has both patient and parent versions. All patients completed the patient version, and parents whose children were assigned to family-supported treatment also completed the parent version. We used the PARDI to determine ARFID diagnosis, as well as to evaluate change in ARFID severity and profile scores, from pre- to post-treatment. We also used the PARDI, supplemented by clinical impression, to assign CBT-AR Stage 3 modules. The PARDI was administered by independent assessors (MCK, JHJ, OBW, ACK) who did not serve as study therapists. Cronbach alphas for PARDI profiles ranged from .72 to .92 in the current sample for both patient and parent ratings. Cronbach alpha for severity was lower (.65 for patients and .57 for parents). Inter-rater reliability of the ARFID diagnosis (yes/no) for 25% of randomly selected cases at both pre- and post-treatment was excellent (100% agreement; kappa = 1.0) for both patient and parent PARDI interviews.
Kiddie Schedule for Affective Disorders and Schizophrenia for School Age Children-Present and Lifetime Version (KSADS-PL).
The KSADS-PL is a semi-structured interview assessing current and lifetime psychiatric diagnoses in children and adolescents (Kaufman et al., 2013). The KSADS-PL Working Draft was updated in 2013 to assess DSM-5 criteria. We used the KSADS-PL at baseline to assess inclusion/exclusion criteria and characterize the sample by diagnosing comorbid psychiatric disorders. Inter-rater reliability for 10% of randomly selected cases at pre-treatment was 100% (kappa = 1.0) for all comorbid diagnoses.
Credibility/Expectancy Questionnaire.
We used the Credibility/Expectancy Questionnaire (Devilly & Borkovec, 2000) immediately after session 1 (in which patients heard the rationale for CBT-AR) to assess patients’ and parents’ confidence in the treatment and expectation for symptom change. We adapted items to refer to “food restriction and avoidance” as target symptoms. For parents, we modified items to refer to “your child.” To calculate credibility, we added the three items regarding how successful the patient thought the treatment would be, how logical it sounded, and how confident they would be recommending it to a friend, and divided by three. Scores ranged from 1 (not at all logical/successful) to 10 (very logical/successful). For expectancy, patients and parents rated the percentage change (0–100%) in the patient’s eating that they thought would occur by the end of treatment.
Client Satisfaction Questionnaire (CSQ).
We used an 8-item version of the CSQ (Attkisson & Zwick; 1982) to assess satisfaction with treatment quality, delivery, and length, and the extent to which the treatment met patient needs. Scores range from 8 to 32. Higher scores indicated greater satisfaction. We gave this measure to all patients at post-treatment (Cronbach alpha .86), as well as to parents (Cronbach alpha .78) in family-supported CBT-AR. For parents, we modified items to refer to “your child.”
Clinical Global Impression Scale (CGI).
The CGI provides a global rating of symptom change with scores ranging from 1 (very much improved) to 7 (very much worse) (Guy, 1976). Therapists provided CGI ratings on their own patients at post-treatment and we interpreted a score of 1 or 2 (much or very much improved, respectively) as representing positive treatment response (Wilhelm et al., 2011).
Food Neophobia Scale (FNS).
The FNS is a 10-item self-report questionnaire that assesses reluctance to try novel foods (Pliner & Hobden, 1992). It is rated on a 7-point Likert scale with anchors ranging from “disagree extremely” to “agree extremely.” Scores range from 10–70, with higher scores representing greater neophobia. Cronbach alpha was .94 for the current sample. We used the FNS to evaluate change in willingness to try novel foods from pre- to post-treatment.
Number of new foods incorporated.
At post-treatment, therapists recorded the number of new foods that their patients had incorporated during the course of CBT-AR. To qualify as new, foods had to be either (a) never previously eaten; or (b) if previously eaten, dropped or refused for several months prior to starting treatment. To qualify as incorporated, patients needed to be routinely eating these foods (approximately once per week) in normative serving sizes outside of treatment sessions (not just tasting small bites of the food in session), based on self-monitoring records.
Height, weight, and menstruation status.
At pre- and post-treatment, a nurse practitioner (KH) measured height in triplicate on a stadiometer and averaged the three height measurements. Weight was measured on a calibrated scale with patients dressed in a hospital gown, pants, and socks which were weighed separately and subtracted from the clothed weight measurement. Height and weight were combined to obtain body mass index (BMI) and compared against age- and sex-matched norms (Kuczmarski et al., 2002) to obtain a BMI percentile. In females, menstruation status was obtained at pre- and post-treatment by asking patients about their menstrual history and dates of last and previous menstrual periods.
State-Trait Anxiety Inventory—Child Version (STAIC) Trait Subscale.
The STAIC trait subscale is a 20-item self-report questionnaire measuring anxiety proneness that is hypothesized to be stable across threatening situations (Spielberger et al., 1983). Items assess how the respondent usually feels and have a 3-response scale. Scores range from 20–80 with higher scores indicating greater anxiety proneness. Cronbach alpha was .85 in the current sample. We used the STAIC trait subscale to evaluate change in anxiety symptoms from pre- to post-treatment.
Child Depression Inventory 2 (CDI-2).
The CDI-2 is a self-report questionnaire that measures symptoms of depression in children (Kovacs, 1992). Scores range from 0 to 56, with higher scores indicating greater depression. Cronbach alpha was .72 in the current sample. We used the CDI-2 to evaluate change in depressive symptoms from pre- to post-treatment.
Data Analysis
To test our hypotheses about feasibility and acceptability, we calculated the percentage of patients offered the treatment who took it up, and the percentage of patients who completed treatment. We also calculated the average number of sessions completed. We then interpreted the pre-treatment credibility and expected improvement ratings for all patients who started treatment, and satisfaction scores for all completers. (We did not obtain post-treatment satisfaction scores for dropouts.)
To test our hypotheses about proof-of-concept, we used intent-to-treat analyses with baseline assessment carried forward for non-completers. Specifically, we evaluated the frequency of positive treatment response at the final session (i.e., CGI scores of “much improved” or “very much improved”). Next, we conducted a series of paired t-tests to evaluate change in pre- versus post-outcome measures, assuming no change in non-completers. We set the significance level at p < .05 and did not correct for family-wise error given the preliminary nature of this study. We calculated within-subjects effect sizes as Cohen’s d, which we interpreted as small (d = .20), medium (d = .50), or large (d = .80). We compared pre- and post-measures of ARFID symptom severity and ARFID profile scores based on both patient- and parent- report, as well as FNS. For those who were underweight, we also compared weight and BMI percentile. We also determined the number of underweight female patients, who were either premenarcheal or had amenorrhea at pre-treatment, who began menstruating by post-treatment. We evaluated the change in the proportion of the sample meeting criteria for ARFID using McNemar’s chi squared tests for dependent categories. Lastly, to evaluate the impact of the treatment on general psychopathology, we evaluated change in STAIC and CDI-2 from pre- to post-treatment. We conducted all analyses using SPSS version 25.
Results
Feasibility and acceptability
Of the 25 individuals whom we offered CBT-AR, 20 chose to take it up. Table 1 presents the pre-treatment demographic and clinical characteristics of the 20 patients who were included in intent-to-treat analyses. Of those, three (15%) dropped out and 17 (85%) completed treatment. One patient dropped out after session 1 because the patient was offered family-supported CBT-AR, but the parents did not wish to take part in treatment. One underweight patient dropped out after session 3 because the parents decided to pursue a partial hospitalization program. A final non-underweight patient stopped attending appointments after session 12 in the context of increased depression. Of those who completed the treatment, underweight patients completed an average of 24.4 (SD = 5.2) sessions. Similarly, patients who were not underweight completed an average of 20.6 (SD = 2.9) sessions. For completers, sessions were delivered over an average of 33.7 (SD = 11.2) weeks. Stage 3 modules were assigned according to the ARFID presentation (or combination of presentations) described in Table 1. All patients and parents, including those who later dropped out, endorsed high ratings of treatment credibility and expectations for improvement after session 1. At post-treatment, patients and their parents who completed the treatment reported high ratings of treatment satisfaction (Table 2).
Table 1.
Pre-treatment demographic and clinical characteristics of 20 patients in an initial proof-of-concept study of cognitive-behavioral therapy for avoidant/restrictive food intake disorder (CBT-AR)
| M (SD) or n (%) | |
|---|---|
| Age (years) | 13.2 (2.1) |
| Gender | |
| Male | 11 (55%) |
| Female | 9 (45%) |
| Race | |
| Caucasian | 18 (90%) |
| Non-Caucasian | 2 (10%) |
| Ethnicity | |
| Hispanic | 1 (5%) |
| Non-Hispanic | 19 (95%) |
| ARFID Presentation(s) | |
| Sensory Sensitivity only | 6 (30%) |
| Lack of Interest only | 1 (5%) |
| Fear of Aversive Consequences only | 3 (15%) |
| Sensory Sensitivity + Lack of Interest | 10 (50%) |
| Sensory Sensitivity + Fear of Aversive Consequences | 0 (0%) |
| Lack of Interest + Fear of Aversive Consequences | 0 (0%) |
| All 3 presentations | 0 (0) |
| DSM-5 criteria met for ARFID (A1-A4) based on patient-rated PARDI | |
| A1 Low weight (BMI < 10th percentile), significant weight loss, and/or failure to grow | 14 (70%) |
| A2 Nutritional deficiency (diagnosed by healthcare professional via blood test) | 2 (10%) |
| A3 Dependence on nutritional supplements (i.e., prescribed vitamins or high-energy drinks) | 8 (40%) |
| A4 Psychosocial impairment (one or more PARDI impairment items ≥ 4) | 12 (60%) |
| Current comorbid psychiatric diagnoses by KSADS-PL* | |
| Panic disorder | 1 (5%) |
| Subthreshold panic disorder | 1 (5%) |
| Social anxiety disorder | 1 (5%) |
| Phobic disorder | 3 (15%) |
| Generalized anxiety disorder | 4 (20%) |
| Obsessive-compulsive disorder | 1 (5%) |
| Attention deficit hyperactivity disorder | 1 (5%) |
| Other specified attention deficit hyperactivity disorder | 1 (5%) |
| No comorbid diagnoses | 13 (65%) |
| Weight status** | |
| Underweight | 14 (70%) |
| BMI percentile | 9.80 (9.1) |
| Normal weight | 6 (30%) |
| BMI percentile | 45.7 (31.8) |
| Eating Disorder Examination-Questionnaire Global | 0.17 (0.29) |
| Treatment format | |
| Family-supported | 18 (90%) |
| Individual | 2 (10%) |
Diagnoses do not add up to 100% because some patients had multiple comorbid diagnoses.
None of the patients in this study were overweight (BMI > 85th percentile) or obese (BMI > 95th percentile). One patient was reclassified during treatment from not underweight to underweight because he grew taller without commensurate weight gain. Accordingly, the therapist prioritized weight gain as a focus for the remainder of the treatment. However, the patient continued in the individual version of the treatment and did not switch to the family-supported version.
Note. ARFID = avoidant/restrictive food intake disorder; PARDI = Pica, ARFID, and Rumination Disorder Interview; KSADS-PL = Kiddie Schedule for Affective Disorders and Schizophrenia for School Age Children-Present and Lifetime Version; BMI = body mass index
Table 2.
Pre- and post-treatment measures of feasibility, acceptability, and clinical outcomes for the intent-to-treat sample (n = 20) in a proof-of-concept study of cognitive-behavioral therapy for avoidant/restrictive food intake disorder (CBT-AR)
| Possible Range on Measure | Pre-treatment M (SD) or n (%) |
Post-treatment M (SD) or n (%) |
t | df | p | Effect size (d) |
|
|---|---|---|---|---|---|---|---|
| Credibility (evaluated after session 1 only) | |||||||
| Patient | 0–10 | 7.9 (1.9) | ___ | ___ | ___ | ___ | ___ |
| Parent* | 0–10 | 7.6 (1.7) | ___ | ___ | ___ | ___ | ___ |
| Expected percent change in ARFID symptoms (evaluated after session 1 only) | |||||||
| Patient | 0–100% | 67.4 (18.2) | ___ | ___ | ___ | ___ | ___ |
| Parent* | 0–100% | 53.5 (16.9) | ___ | ___ | ___ | ___ | ___ |
| Client Satisfaction Questionnaire (evaluated at post-treatment only)** | |||||||
| Patient | 8–32 | ___ | 30.0 (3.0) | ___ | ___ | ___ | ___ |
| Parent* | 8–32 | ___ | 30.0 (2.1) | ___ | ___ | ___ | ___ |
| Therapist rating of patient as “much improved” or “very much improved” on Clinical Global Impression—Global Improvement Scale | 0–100% | ___ | 17 (85%) | ___ | ___ | ___ | ___ |
| Pica, ARFID, and Rumination Disorder Interview—Patient Ratings | |||||||
| Overall Severity | 0–6 | 2.4 (0.7) | 1.2 (1.0) | 5.19 | 19 | <.001 | 1.18 |
| Sensory Sensitivity | 0–6 | 1.1 (1.0) | 0.7 (1.1) | 2.76 | 19 | .012 | 0.63 |
| Lack of Interest | 0–6 | 1.4 (1.4) | 0.7 (0.8) | 2.35 | 19 | .030 | 0.55 |
| Fear of Aversive Consequences | 0–6 | 0.5 (1.0) | 0.0 (0.1) | 2.07 | 19 | .052 | 0.49 |
| Pica, ARFID, and Rumination Disorder Interview—Parent Ratings* | |||||||
| Overall Severity | 0–6 | 2.8 (0.6) | 1.8 (1.0) | 4.53 | 16 | <.001 | 1.12 |
| Sensory Sensitivity | 0–6 | 2.0 (1.2) | 1.5 (1.4) | 2.18 | 16 | .045 | 0.46 |
| Lack of Interest | 0–6 | 1.9 (1.5) | 1.5 (1.4) | 1.61 | 16 | .127 | 0.35 |
| Fear of Aversive Consequences | 0–6 | 0.8 (1.0) | 0.3 (0.7) | 2.15 | 16 | .047 | 0.59 |
| Food Neophobia Scale | 10–70 | 53.0 (14.5) | 43.8 (15.5) | 3.28 | 19 | .004 | 0.73 |
| Number of new foods incorporated | __ | __ | 16.7 (12.1) | __ | __ | __ | |
| Weight status—Underweight patients only | |||||||
| Weight (lbs) | ____ | 84.1 (18.0) | 95.6 (18.7) | −7.14 | 13 | <.001 | 1.91 |
| BMI percentile | 0–100 | 9.80 (9.1) | 19.6 (15.2) | −4.86 | 13 | <.001 | 1.29 |
| Met diagnostic criteria for ARFID on Pica, ARFID, and Rumination Disorder Interview | |||||||
| Patient Ratings | % of 20 patients | 19 (95%) | 6 (30%) | <.001 | __ | ||
| Parent Ratings* | % of 17 patients | 17 (100%) | 7 (41%) | .002 | __ | ||
| Comorbid Psychopathology | |||||||
| STAIC – Trait | 20–80 | 29.7 (6.7) | 27.7 (6.5) | 1.88 | 19 | .076 | 0.42 |
| CDI-2 | 0–56 | 6.4 (4.4) | 5.9 (4.6) | 0.65 | 19 | .525 | 0.15 |
We collected parent ratings only for patients assigned to the family-supported version of CBT-AR. Although 18 patients received the family-supported version of the treatment, one set of parents did not complete assessments, so parent ratings are based on n = 17.
Because the Client Satisfaction Questionnaire (CSQ) was administered at post-treatment, CSQ data are only available for completers (n = 17).
Proof-of-concept
Table 2 displays pre- and post-treatment scores on all outcome measures. Therapists rated all 17 completers as “much improved” or “very much improved” on the CGI. Two non-completers were rated as having “no change” and a third as “minimally improved.” Across the whole sample, PARDI patient ratings of ARFID overall severity (p < .001), sensory sensitivity (p = .012), and lack of interest (p = .030) decreased significantly with medium to large effect sizes. Patient ratings of PARDI fear of aversive consequences decreased at trend-level (p = .052) with a medium effect size. For those in the family-supported version, parent-rated PARDI overall severity (p < .001), PARDI sensory sensitivity (p = .045), and PARDI fear of aversive consequences (p = .047) decreased significantly with medium to large effect sizes, whereas parent-rated PARDI lack of interest did not change. Food neophobia decreased significantly (p = .004) with a medium to large effect size. Patients incorporated a mean of 16.7 (SD = 12.1) new foods from pre- to post-treatment, primarily from groups under-represented in their diets including fruits, vegetables, and proteins. The underweight subgroup (n = 14) gained an average of 11.5 (SD = 6.0) pounds from pre- to post-treatment (p < .001), moving from approximately the 10th percentile to the 20th percentile for BMI (p < .001), representing a significant increase with a large effect size. Of the five females who were underweight and not menstruating at pre-treatment, three had begun menstruating by post-treatment. Using the PARDI diagnostic algorithm, patients were significantly less likely to meet criteria for ARFID at post-treatment, by both patient (p < .001) and parent (p = .002) report. Regarding co-occurring difficulties, trait anxiety decreased at trend level with a small effect size, but depression scores did not change.
Adverse events
There were two adverse events, both during Stage 3. In the first adverse event, rated as grade 1 (mild) and related to the protocol, a patient vomited involuntarily during an in-session interoceptive exposure (i.e., drinking several cups of water to experience fullness) in the lack of interest module. In a second adverse event, rated as grade 1–2 (mild to moderate) and related to the protocol, a patient experienced mouth tingling after tasting nuts during a session in the sensory sensitivity module. We referred the second patient to the emergency department where the patient was given epinephrine and diagnosed with a nut allergy. Both patients went on to complete CBT-AR.
Discussion
Our initial study of 20 patients provides preliminary evidence for feasibility, acceptability, and proof-of-concept for CBT-AR in an outpatient sample of relatively mild severity. Specifically, the majority of patients offered the treatment elected to take it up, and few patients dropped out. Patients and families rated CBT-AR as credible upon starting treatment and satisfactory upon completing it. Therapists, patients, and parents reported significant improvements on multiple measures of ARFID symptoms, ranging from the incorporation of new foods to significant gains in weight. Perhaps most notably, 70% of patients no longer met criteria for ARFID at the end of treatment. Taken together, findings suggest that CBT-AR may be a promising new treatment for children and adolescents with ARFID. Further research with larger samples, randomized designs, more severely ill populations, and post-treatment follow-up assessments are required to more rigorously evaluate treatment efficacy.
Our findings are consistent with a recent case series reporting that another form of CBT for ARFID (delivered in an intensive four-week day-hospital format) was effective in reducing ARFID symptoms in adolescents (Dumont et al., 2019). However, our results extend upon the prior study by demonstrating positive effects of CBT in a larger sample and highlighting that CBT may lead to significant symptom reduction even when delivered in a less time-intensive and more cost-effective weekly outpatient format. Furthermore, our results suggest that it is possible to treat ARFID successfully with only a therapist and physician, and without a large multidisciplinary team. Given that the majority of patients in our study received the family-supported version of CBT-AR, our findings are also consistent with recent case series (Lock et al., 2019b; Rienecke et al., 2020) and one pilot RCT of young children (Lock et al., 2019a) that have reported positive effects of FBT for ARFID.
Of course, not all patients responded. Three dropped out and 30% continued to meet criteria for ARFID. The fact that average post-treatment PARDI scores were still slightly higher than those observed in a prior study of healthy controls suggests that patients remained somewhat symptomatic after treatment completion (Bryant-Waugh et al., 2019). It is notable that, by both patient- and parent- report, ARFID severity and sensory sensitivity showed significant reductions, whereas patients (but not parents) reported significant reductions in lack of interest in eating or food. It is possible that while CBT-AR helps patients make the necessary behavioral changes to increase food intake, parents need to continue to support their children in eating sufficient amounts even upon treatment completion. Indeed, available data suggest that the low appetite reported by those with the lack of interest presentation of ARFID may be neurobiologically mediated. In one study, low-weight girls with ARFID showed significantly lower levels of the orexigenic appetite-stimulating hormone ghrelin than girls with anorexia nervosa, both while fasting and in response to a test meal (Becker et al., under review).
Of note, neither anxiety nor depression changed significantly during the course of treatment. However, the average pre-treatment STAIC and CDI-2 scores in our sample were near the mean for healthy individuals, suggesting little room for improvement. Given the well-known relationship between depression and low appetite, it is interesting that both depression and parent-reported lack of interest showed similarly stable trajectories that were unaffected by the intervention. Future studies might explore comorbid disorders as moderators of treatment outcome in behavioral interventions for ARFID.
The two adverse events have implications for practicing clinicians. Our results highlight the potential for vomiting when conducting interoceptive exposures with patients who endorse nausea and post-prandial fullness. Moreover, clinicians who treat patients with ARFID should be aware of the potential for allergic reactions when presenting novel foods to individuals with long histories of selective eating. Of note, both patients who experienced adverse events remained in the treatment, suggesting that they may have been willing to experience some discomfort for the possibility of reducing ARFID symptoms.
Our study should be interpreted in light of limitations. First, because there was no control group, we cannot rule out alternative rival hypotheses (e.g., that the passage of time, or therapist attention, was responsible for symptom improvement). Second, the sample size was small, so p-values and effect sizes must be interpreted with caution. Third, pre-treatment PARDI scores were fairly low, which may limit generalizability to those with more severe presentations. Of note, low PARDI scores in the current study may have been due to the outpatient level of care, lack of patient insight into symptom severity (given that parents provided higher scores than did children), and the heterogeneity of ARFID presentations which resulted in some patients endorsing high PARDI scores consistent with their primary ARFID presentation (e.g., sensory sensitivity) but low scores on the others (e.g., lack of interest, fear of aversive consequences). Fourth, because we recruited our sample from a larger neurobiology study of ARFID, patients had to meet some inclusion criteria (e.g., ability to complete MRI) that would not normally be applied in routine clinical care and were offered monetary compensation for completing assessments, which could limit generalizability of the findings. Lastly, only two of the 20 participants received the individual version of CBT-AR, which limits the conclusions that can be drawn about proof-of-concept for this therapeutic format in youth.
The study also had important strengths. First, therapists delivered CBT-AR according to a published manual and exhibited high levels of treatment fidelity as confirmed by an independent rater. Second, the primary outcome measure—the PARDI interview—was conducted by assessors who were not therapists in the current study. Third, CBT-AR was associated with symptom improvement across multiple informants (i.e., therapists, patients, and parents). Fourth, the use of intent-to-treat analyses, which assumed no change among non-completers, provided a conservative test of our hypotheses.
In conclusion, CBT-AR demonstrated initial evidence of feasibility, acceptability, and proof-of-concept for children and adolescents in this uncontrolled trial. To our knowledge, this is the first study of an outpatient psychosocial treatment for ARFID in older adolescents. The ORBIT model of intervention development suggests that successful proof-of-concept studies be followed up with randomized controlled trials (Czajkowski et al., 2015). Thus larger studies are needed to compare CBT-AR to a comparator condition (e.g., wait list, usual care, supportive therapy, or nutrition counseling) to replicate and extend the current findings and to provide a formal test of treatment efficacy.
Acknowledgements:
This study was funded by the Hilda & Preston Davis Foundation, American Psychological Foundation, Global Foundation for Eating Disorders, and NIMH R01MH108595 (PIs Thomas/Lawson/Micali).
Footnotes
Conflicts of interest: Drs. Thomas and Eddy receive royalties from Cambridge University Press for the sale of their book Cognitive-Behavioral Therapy for Avoidant/Restrictive Food Intake Disorder: Children, Adolescents, and Adults. Drs. Thomas, Eddy, and Becker will receive royalties for the sale of their upcoming self-help book on cognitive-behavioral therapy for ARFID.
Presentation information: Preliminary findings from this study were presented as an oral abstract at the Eating Disorders Research Society annual meeting in Sydney, Australia (October 25–27, 2018).
Data availability statement: Data from this study are available from the first author upon reasonable request.
References
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders. 5th Ed. Arlington, VA: American Psychiatric Association. [Google Scholar]
- Attkisson CC, & Zwick R (1982). The Client Satisfaction Questionnaire: Psychometric properties and correlations with service utilization and psychotherapy outcome. Evaluation and Program Planning, 5, 233–237. [DOI] [PubMed] [Google Scholar]
- Aulinas A, Marengi D, Galbiati F, Asanza E, Slattery M, Mancuso CJ, Wons O, Micali N, Bern E, Eddy KT, Thomas JJ, Misra M, & Lawson EA (2020). Medical comorbidities and endocrine dysfunction in low-weight females with avoidant/restrictive food intake disorder compared to anorexia nervosa and healthy controls. International Journal of Eating Disorders, 53(4), 631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker KR, Keshishian AC, Liebman RE, Coniglio KA, Wang SB, Franko DL, Eddy KT, & Thomas JJ (2019). Impact of expanded diagnostic criteria for avoidant/restrictive food intake disorder on clinical comparisons with anorexia nervosa. International Journal of Eating Disorders, 52, 230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker KR, Mancuso C, Azanza E, Izquierdo A, Wons O, Dreier M, Slattery M, Plessow F, Micali N, Eddy KT, Thomas JJ, Misra M, & Lawson EA Distinct ghrelin and PYY response in adolescent girls with low-weight avoidant/restrictive food intake disorder compared to anorexia nervosa and healthy controls. Under review.
- Bryson AE, Scipioni AM, Essayli JH, Mahoney JR, & Ornstein RM Outcomes of low-weight patients with avoidant/restrictive food intake disorder and anorexia nervosa at long-term follow-up after treatment in a partial hospitalization program for eating disorders. (2018). International Journal of Eating Disorders, 51, 470–474. [DOI] [PubMed] [Google Scholar]
- Bryant-Waugh R, Micali N, Cooke L, Lawson EA, Eddy KT, & Thomas JJ (2019). The Pica, ARFID, and Rumination Disorder Interview: Development of a multi-informant, semi-structured interview of feeding disorders across the lifespan: A pilot study for ages 10–22. International Journal of Eating Disorders, 52, 378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czajkowski SM, Powell LH, Adler N,, Naar-King S, y KD, Hunter CM, Laraia B, Olster DH, Perna FM, Peterson JC, Epel E, Boyington JE, & Charlson ME (2015). From ideas to efficacy: The ORBIT model for developing behavioral treatments for chronic diseases. Health Psychology, 34(10), 971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlsgaard KK, & Bodie J (2019). The (extremely) picky eaters clinic: A pilot trial of a seven-session group behavioral intervention for parents of children with avoidant/restrictive food intake disorder. Cognitive and Behavioral Practice, 26(3);492–505. [Google Scholar]
- Devilly GJ, & Borkovec TD (2000). Psychometric properties of the credibility expectancy questionnaire. Journal of Behavior Therapy and Experimental Psychiatry, 31, 73–86. [DOI] [PubMed] [Google Scholar]
- Dumont E, Jansen A, Kroes D, de Haan E, & Mulkens S (2019). A new cognitive behavior therapy for adolescents with avoidant/restrictive food intake disorder in a day treatment setting: A clinical case series. International Journal of Eating Disorders, 52, 447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt S, Martell C, Lowe KD, Le Grange D, & Ehrenreich-May J (2019). An ARFID case report combining family-based treatment with the unified protocol for transdiagnostic treatment of emotional disorders in children. Journal of Eating Disorders, 7(1); 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairburn CG (2008). Cognitive Behavior Therapy and Eating Disorders. New York: Guilford Press; 2008a. [Google Scholar]
- Fairburn CG, & Beglin S (2008). Eating Disorder Examination-Questionnaire (EDE-Q 6.0) In Cognitive Behavior Therapy and Eating Disorders (Fairburn CG, Ed.), Guilford Press, New York, pp. 309–313. [Google Scholar]
- Fitzpatrick KK, Forsberg SE, & Colborn D (2015). Family-based therapy for avoidant restrictive food intake disorder: Families facing food neophobias In Loeb KL, Le Grange D, & Lock J (Eds.), Family therapy for adolescent eating and weight disorders: New applications (pp. 256–276). New York: Routledge. [Google Scholar]
- Görmez A, Kılıç A, & Kırpınar İ (2018). Avoidant/restrictive food intake disorder: An adult case responding to cognitive behavioral therapy. Clinical Case Studies, 17, 443–452. [Google Scholar]
- Guy W (1976). ECDEU assessment manual for psychopharmacology. Washington, DC: United States Department of HEW Publications. [Google Scholar]
- Hunter PV, & Antony MM (2009). Cognitive-behavioral treatment of emetophobia: The role of interoceptive exposure. Cognitive and Behavioral Practice, 16, 84–91. [Google Scholar]
- Kambanis PE, Kuhnle MC, Wons OB, Jo JH, Keshishian AC, Hauser K, Becker KR, Franko DL, Misra M, Micali N, Lawson EA, Eddy KT, & Thomas JJ (2020). Prevalence and correlates of psychiatric comorbidities in children and adolescents with full and subthreshold avoidant/restrictive food intake disorder. International Journal of Eating Disorders. 53(2), 256–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Axelson D, Perepletchikova F, Brent D, & Ryan N (2013). Kiddie Schedule for Affective Disorders and Schizophrenia (KSADS-PL). Working draft/unpublished interview.
- Kovacs M (1992). Children’s Depression Inventory: Manual (p. Q8). North Tonawanda, NY: Multi-Health Systems. [Google Scholar]
- Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. (2002). CDC growth charts for the United States: Methods and development. Vital and Health Statistics, 11, 246, 1–190. [PubMed] [Google Scholar]
- Kurz S, Van Dyck Z, Dremmel D, Munsch S, & Hilbert A (2015). Early-onset restrictive eating disturbances in primary school boys and girls. European Child & Adolescent Psychiatry, 24, 779–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock J, Sadeh-Sharvit S, & L’Insalata A (2019a). Feasibility of conducting a randomized clinical trial using family-based treatment for avoidant/restrictive food intake disorder. International Journal of Eating Disorders, 52, 746–751. [DOI] [PubMed] [Google Scholar]
- Lock J, Robinson A, Sadeh-Sharvit S, Rosania K, Osipov L, Kirz N, et al. (2019b). Applying family-based treatment (FBT) to three clinical presentations of avoidant/restrictive food intake disorder: Similarities and differences from FBT for anorexia nervosa. International Journal of Eating Disorders, 52, 439–446. [DOI] [PubMed] [Google Scholar]
- Makhzoumi SH, Schreyer CC, Hansen JL, Laddaran LA, Redgrave GW, & Guarda AS (2019). Hospital course of underweight youth with ARFID treated with a meal-based behavioral protocol in an inpatient-partial hospitalization program for eating disorders. International Journal of Eating Disorders, 52, 428–434 [DOI] [PubMed] [Google Scholar]
- McNally RJ (1994). Choking phobia: a review of the literature. Comprehensive Psychiatry, 35, 83–89. [DOI] [PubMed] [Google Scholar]
- Nicely TA, Lane-Loney S, Masciulli E, Hollenbeak CS, & Ornstein RM (2014). Prevalence and characteristics of avoidant/restrictive food intake disorder in a cohort of young patients in day treatment for eating disorders. Journal of Eating Disorders, August 2, 2(1), 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris ML, Robinson A, Obeid N, Harrison M, Spettigue W, & Henderson K (2014). Exploring avoidant/restrictive food intake disorder in eating disordered patients: A descriptive study. International Journal of Eating Disorders, 47, 495–499. [DOI] [PubMed] [Google Scholar]
- Pliner P, & Hobden K (1992). Development of a scale to measure the trait of food neophobia in humans. Appetite, 19, 105–120. [DOI] [PubMed] [Google Scholar]
- Rienecke RD, Drayton A, Richmond RL, & Mammel KA (2020). Adapting treatment in an eating disorder program to meet the needs of patients with ARFID: Three case reports. Clinical Child Psychology and Psychiatry, 25(2), 293–303. [DOI] [PubMed] [Google Scholar]
- Sharp WG, Allen AG, Stubbs KH, Criado KK, Sanders R, McCracken CE, et al. (2017). Successful pharmacotherapy for the treatment of severe feeding aversion with mechanistic insights from cross-species neuronal remodeling. Translational Psychiatry, 7(6), e1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp WG, Stubbs KH, Adams H, Wells BM, Lesack RS, Criado KK, et al. (2016). Intensive, Manual-based intervention for pediatric feeding disorders: results from a randomized pilot trial. Journal of Pediatric Gastroenterology and Nutrition, 62, 658–663. [DOI] [PubMed] [Google Scholar]
- Spettigue W, Norris ML, Santos A, & Obeid N (2018). Treatment of children and adolescents with avoidant/restrictive food intake disorder: a case series examining the feasibility of family therapy and adjunctive treatments. Journal of Eating Disorders, 6(1), 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, & Jacobs GA (1983). Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Steen E, & Wade TD (2018). Treatment of co-occurring food avoidance and alcohol use disorder in an adult: Possible avoidant restrictive food intake disorder? International Journal of Eating Disorders, 51, 373–377. [DOI] [PubMed] [Google Scholar]
- Thomas JJ, Brigham KS, Sally ST, Hazen EP, & Eddy KT (2017). Case records of the Massachusetts General Hospital: An 11-year-old girl with difficulty eating after a choking incident. New England Journal of Medicine, 376(24), 2377–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JJ, & Eddy KT (2019). Cognitive-Behavioral Therapy for Avoidant/Restrictive Food Intake Disorder: Children, Adolescents, and Adults. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Waller G, Turner H, Tatham M, Mountford VA, & Wade TD (2019). Brief Cognitive Behavioural Therapy for Non-underweight Patients: CBT-T for Eating Disorders. Routledge. [Google Scholar]
- Wilhelm S, Phillips KA, Fama JM, Greenberg JL, & Steketee G (2011). Modular cognitive-behavioral therapy for body dysmorphic disorder. Behavior Therapy, 42, 624–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KE, & Foxx RM (2007). Treating Eating Problems of Children with Autism Spectrum Disorders and Developmental Disabilities: Interventions for Professionals and Parents. Austin, Texas: Pro-ed. [Google Scholar]
- Zucker NL, LaVia MC, Craske MG, Foukal M, Harris AA, Datta N, et al. , (2019). Feeling and body investigators (FBI): ARFID division—An acceptance‐based interoceptive exposure treatment for children with ARFID. International Journal of Eating Disorders, 52, 466–472. [DOI] [PMC free article] [PubMed] [Google Scholar]


