Figure 3.
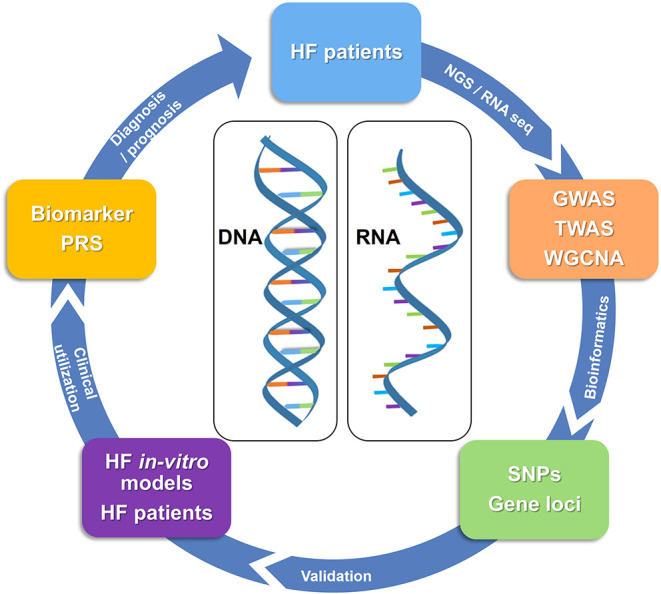
Identification and validation of genetic biomarkers in HF. In order to identify genetic biomarkers, samples taken from HF patients are sequenced and analyzed via GWAS, TWAS as well as WGCNA. Results are then further processed using bioinformatics providing information on SNPs or gene loci associated with HF. Validation of these SNPs by in-vitro models for HF and HF patients provides concrete evidence on the clinical utilization of genetic biomarkers or PRS. Ultimately, genetic biomarkers can be used for diagnosis/prognosis of HF patients in the future. DNA, deoxyribonucleic acid; GWAS, genome-wide association studies; NGS, next generation sequencing; PRS, polygenic risk score; RNA, ribonucleic acid; RNA seq, RNA sequencing; SNPs, single nucleotide polymorphisms; TWAS, transcriptome-wide association studies; WGCNA, weighted gene co-expression network analyses.
