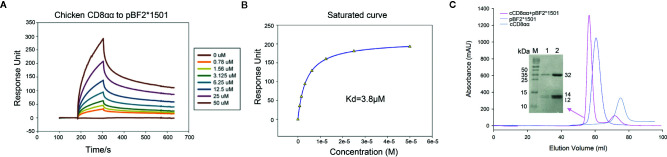
Figure 1.
Affinity measurement by surface plasmon resonance (SPR) and gel column coexistence testing of cCD8αα/pBF2*1501 complex in vitro. (A) pBF2*1501 was the stationary phase, and cCD8αα diluted to 0, 0.78, 1.56, 3.125, 6.25, 12.5, 25, and 50 μM was the mobile phase. (B) The affinity was measured to be Kd=3.8 μM. (C) Purification peak map of cCD8αα, pBF2*1501 and cCD8αα/pBF2*1501, which are colored gray, light blue and pink. The peak position of cCD8αα/pBF2*1501 is in front of that of pBF2*1501, and SDS-PAGE identification of the cCD8αα/pBF2*1501 complex peak shows three distinct bands corresponding to BF2*1501, β2m, and cCD8α protein.