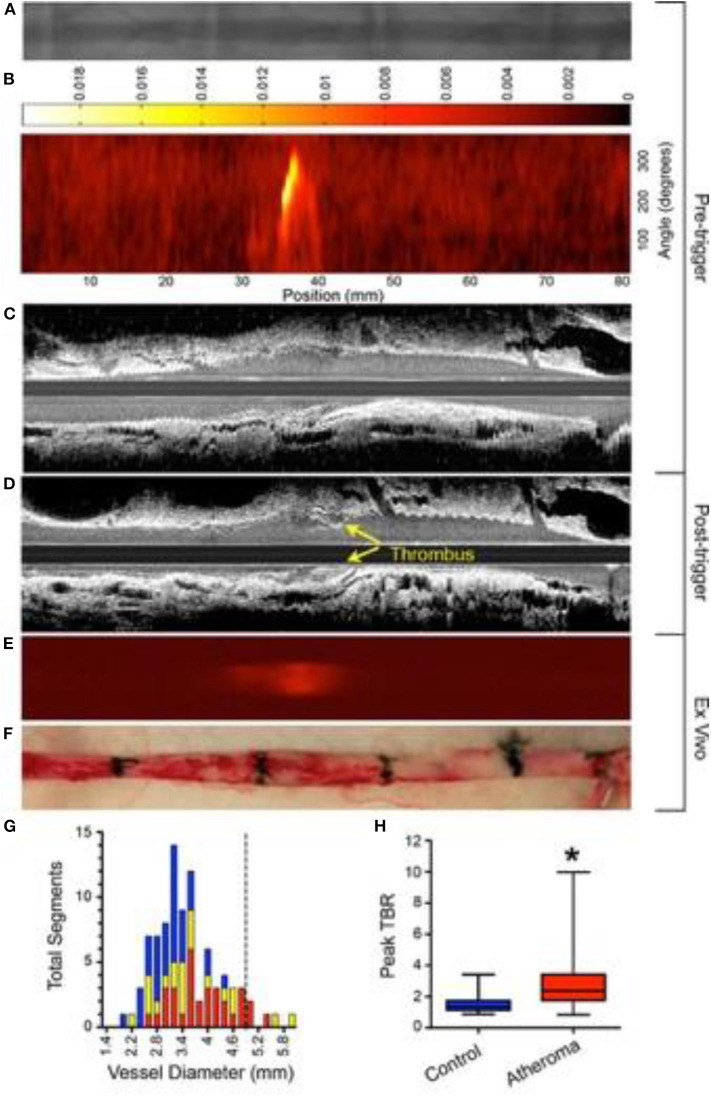
Figure 3.
Experimental in vivo and ex vivo near-infrared fluorescence (NIRF) imaging of inflammation and subsequent atherothrombosis. Rabbits underwent balloon injury at week 2, and were fed high cholesterol diet till week 8. Ten weeks after balloon injury, rabbits received CLIO-CyAm7 (a inflammation-sensitive nanoparticle-based fluorophore), and underwent pharmacologic-triggered plaque thrombosis 24 h later. (A) Pre-trigger x-ray angiography showing the aorta for image coregistration. (B) Pre-trigger in vivo NIRF imaging projected into a 2-dimensional (2D) matrix showing an area of increased signal between 30 and 40 mm. (C,D) Pre- and post-trigger intravascular ultrasound (IVUS) imaging demonistrating induced luminal thrombus (yellow arrows) corresponding to the region of increased NIRF signal intensity on pretrigger NIRF imaging in (B). (E,F) Ex vivo fluorescence reflectance imaging of cross-linked iron oxide (CLIO)-CyAm7 verifying in vivo 2D NIRF imaging, and gross pathology of the resected aorta with 1.5 cm black tissue markings for histological analysis and coregistration. (G) Histogram of vessel diameter measured by cross-sectional IVUS imaging. Red indicates atheroma without attached thrombus, yellow indicates atheroma with attached thrombus, and blue indicates uninjured control aortic segments. The dashed line indicates the 5 mm cut-off for exclusion of NIRF imaging data because of distance attenuation of the NIRF signal in large vessels. (H) In vivo 2D NIRF imaging revealed significantly higher target/background ratio (TBR) in areas with atheroma, compared with uninjured segments of the aorta (peak TBR 2.86 ± 1.82 and 1.55 ± 0.65, *P = 0.001). Stein-Merlob et al. (55), by permission of American Heart Asssociation (AHA). License number: 4720560395030.